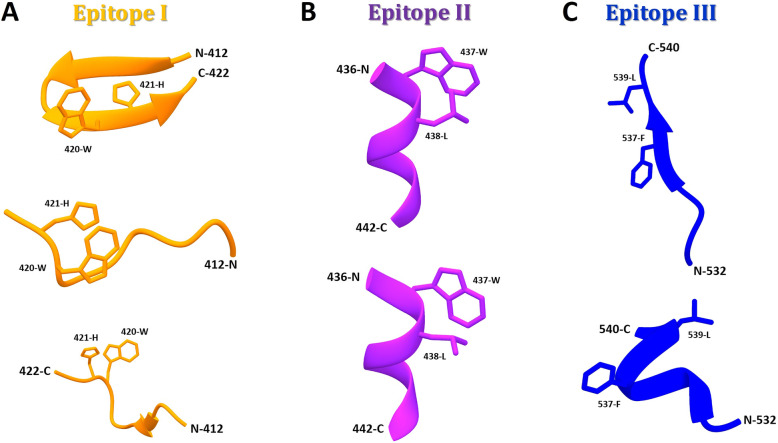
Fig. 2.
E2 epitopes (I to III) adopt distinct conformations when complexed with different antibodies. a Conformations of epitope I: (Upper) Closed β-hairpin in complex with nAb HCV1 (PDB: 4DGY); (Middle) extended-coil conformation in complex with nAb 3/11 (PDB: 4WHY); and (Lower) intermediate-coil conformation with an anti-parallel β-sheet in complex with nAb HC33.1 (PDB:4XVJ). b Conformations of epitope II: (Upper) Preferred state of E2 in the viral particle containing a short 1.5-turn α-helix (aa437–442) and an extended conformation (aa443–446, not shown) in complex with nAb HC84.27 (PDB: 4JZO); and (Lower) conformational changes through short α-helix flipping out (‘open state’) to expose aa437(W) and aa438(L) residues for nAb mAb#8 (PDB: 4HZL). c Conformations of epitope III: (Upper) Open and stabilized strand in β-sheet conformation with aa537(F) and aa539(L) residues flipped out into the hydrophobic part of the Ig-like domain in complex with AR3C Fab (PDB: 4MWF); and (Lower) helical disordered conformation with aa537(F) and aa539(L) residues solvent-exposed in complex with non-nAb DAO5 (PDB: 5NPJ)