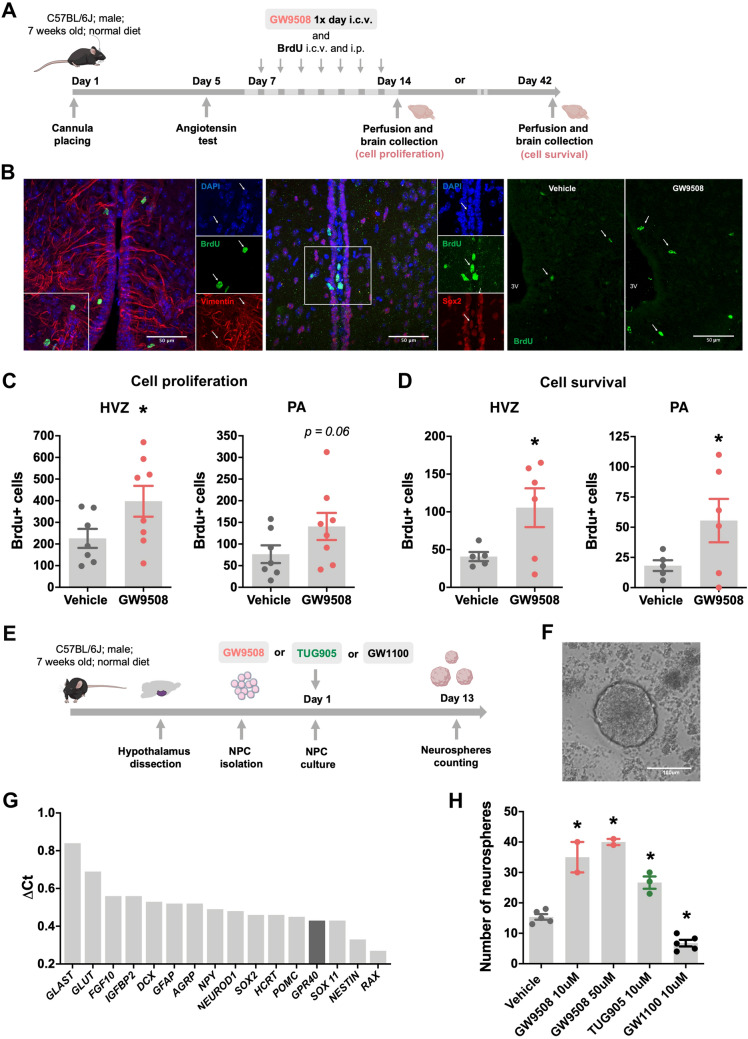
Figure 1.
GPR40 modulates cell proliferation and survival in the hypothalamus of adult mice. C57BL/6J mice received a 7-day repeated treatment of GW9508 or vehicle, and BrdU, were sacrificed 24 h or 28 days after the last BrdU injection by transcardial perfusion and their brains were processed for immunohistochemistry (A). The co-labeling of BrdU/vimentin and BrdU/sox2 positive cells indicates the neural precursor phenotype of newborn cells after 24 h of BrdU injections (B). Panel B also shows representative images of BrdU-positive cells in the hypothalamic ventricular zone (HVZ) of vehicle and GW9508 treated mice after 24 h. The GW9508 treated mice showed increased number of BrdU immunopositive cells in the in the HVZ (C). Immunolabeling for BrdU-positive cells present in the hypothalamus 28 days after the last BrdU administration reveals higher survival of newborn cells in both the HVZ and parenchyma (PA) of GW9508 treated mice (D). White arrows indicate either BrdU, vimentin and sox2 immunopositive cells in the HVZ. Scale bars = 50 μm (B). The effect of GPR40 over adult NPC proliferation was also assessed ex vivo. Cell proliferation was estimated by quantifying the number of primary neurospheres generated after 13 days exposure to GPR40 agonists (GW9508 and TUG905) and antagonist (GW1100) (E). Phase-contrast image of hypothalamic neurosphere generated from adult hypothalamic NPC cells and cultured with growth factors in non-adhesive conditions (F). Neurospheres obtained from control group showed high mRNA expression of NPC markers and hypothalamus-related genes (G). GPR40 activation increased the number of generated neurospheres, while its inhibition reduced NPC proliferation (H). Scale bar = 100 μm (F). Data are presented as means ± SEM. N = 5–7 per group (C, D) 1 (G) and 2–5 preparations (H). *p < 0.05, t-test (C, D); one-way ANOVA followed by Tukey’s post hoc test (H).