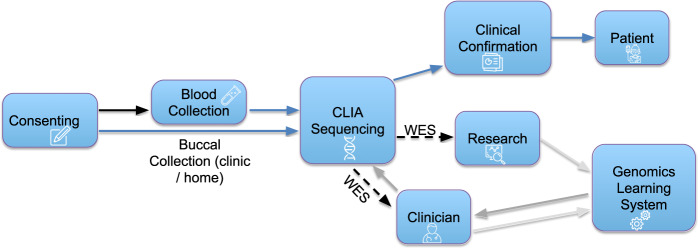
Fig. 2. Research to clinical workflow.
Patients with or without previous clinical testing were consented to harmonized research protocols. Patients were offered standardized sample collection mechanisms and most patients were dual consented to the Precision Link Biobank to support the collection of additional leftover clinical samples. Patient samples were CLIA sequenced by our sequencing provider (GeneDx) and data was returned to AWS where it was loaded into CRDC infrastructure for analysis. Once research teams identified a candidate variant, analysts worked with clinicians to order the clinical confirmation from the sequencing provider. Clinical confirmations were returned to BCH, added to the patient’s medical record, and communicated to the patient.