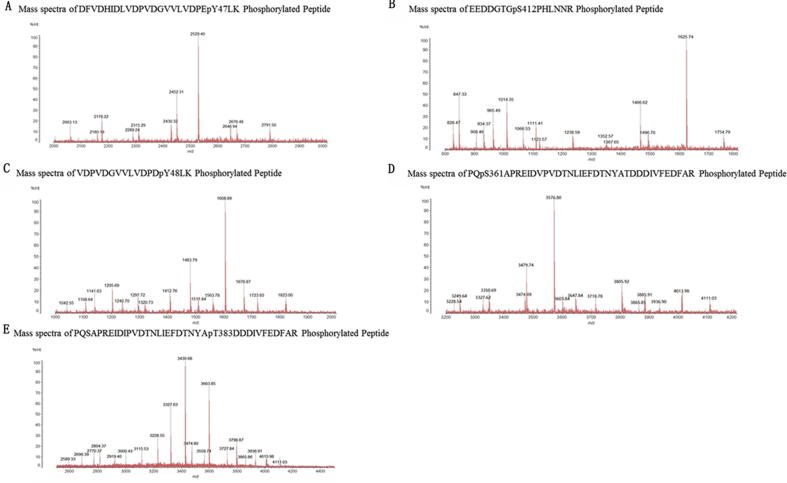
Fig. S5.
Mass spectrometry was used to identify APJ or APJ mutants and β-arrestin interactions to induce β-arrestin phosphorylation sites.
Mass spectrometry identified two phosphorylation sites in β-arrestin1 (Tyr 47 and Ser 412). Mass spectra of DFVDHIDLVDPVDGVVLVDPEpY47LK and EEDDGTGpS412PHLNNR phosphorylated peptides identified from β-arrestin1 were transiently co-expressed with APJ or APJ mutants in HEK-293 cells and immunoprecipitated using β-arrestin1 antibody (Fig. S5 A, B).
Mass spectrometry identified three phosphorylation sites in β-arrestin 2 (Tyr 48, Ser 361 and Thr383). Mass spectra of VDPVDGVVLVDPDpY48LK, PQpS361APREIDVPVDTNLIEFDTNYATDDDIVFEDFAR and PQSAPREIDIPVDTNLIEFDTNYApT383DDDIVFEDFAR phosphorylated peptides identified from β-arrestin2 transiently co-expressed with APJ or APJ mutants in HEK-293 cells and immunoprecipitated using the β-arrestin 2 antibody (Fig. S5 C, D, E).