Abstract
Objective. To systematically assess previous findings on the orbitofrontal sulcogyral pattern in psychiatric disorders and to address the utility of this pattern as a transdiagnostic trait marker of early neurodevelopment in the social brain. Methods. An online literature search was conducted using the PubMed database from inception to August 2019. Studies included in this review were based on the Chiavaras’s original classification method of this H-shaped sulcus (type I, II, and III), intermediate orbital sulcus (IOS), and posterior orbital sulcus (POS). Results. Twenty-six studies were included in the review. Sixteen studies (62%) focused on schizophrenia spectrum (Sz) disorders, and the remaining studies focused on autism spectrum disorder (ASD), attention-deficit/hyperactivity disorder (ADHD), history of extremely preterm and extremely low birth weight, bipolar disorder (BD), panic disorder, obsessive-compulsive disorder, cannabis users, and pathological gambling. In Sz, compared with healthy controls, the orbitofrontal sulcogyral pattern was decreased in type I, increased in type II and III, and there were fewer numbers of IOS and POS reported, although specificity in sex and hemispheric dominance was not consistent. BD and neurodevelopmental disorders in ASD and ADHD showed a similar pattern of alteration to that observed in the Sz. Conclusions. The present review of the orbitofrontal sulcogyral pattern indicated that type I expression might reflect a neurodevelopmental protective marker, and type II and III expressions, as well as fewer numbers of IOS and POS, might reflect neurodevelopmental risk markers. These trait markers may be transdiagnostic among socially disabling diseases.
Keywords: schizophrenia, sulcogyral pattern, orbitofrontal cortex, magnetic resonance imaging, neurodevelopment
Introduction
Early neurodevelopment of the social brain may play an important role in a wide range of psychiatric disorders, including schizophrenia spectrum and autism spectrum disorders. The acquired social deficit consequences induced by a penetrating injury through the orbitofrontal cortex (OFC) have long been known,1 although the connection between anatomical variations of the OFC development with social disabilities have not been well documented. Moreover, and dating back to the seminal work of Bleuler,2 social disturbances in schizophrenia have often been described, but the extent to which they may reflect disease-related neuropathology of the OFC has yet to be established.
OFC, a major part of the social brain, is important for sensory-visceromotor multimodal integration,3 for emotional processing, and for hedonic experience,4 as well as for the evaluation of reinforcers, expectation, motivation, decision making, and goal-directed behavior.5-7 Of particular note, human OFC is characterized by marked interindividual variability in its cytoarchitecture3 and sulcogyral morphology.8-10 The neurodevelopmental formation of the convolutional sulcogyral morphology, which is termed gyrogenesis or gyrification, could reflect neuronal migration, local neuronal connection, synaptic development, and/or lamination of the cortical cytoarchitecture.11,12 In social neuroscience, OFC figures significantly in emotions, decision making, and social behavior, and individual variability in OFC development may therefore be associated with individual variabilities in personality traits, emotional processing, and social behavior.
In order to estimate the degree of cortical folding, a number of the previous studies have employed the gyrification index (GI),13 which is the ratio of the inner and outer cortical surface contours, or more recently the local gyrification index (LGI),14 which considers the 3-dimensional nature of the cortical surface. However, neither GI nor LGI are independent of brain tissue volume, and thus they are potentially unstable longitudinally and susceptible to confounds affecting brain tissue volume. Given that the sulcogyral morphology is genetically determined,15 and is formed during early neurodevelopment and then stable over time, the sulcogyral pattern classification may provide a morphological trait marker, which could reflect neurodevelopmental variability independent of brain tissue volumes, their longitudinal changes, and possibly confounding factors, including psychotropic medications and chronic illness. Accordingly, the categorical sulcogyral pattern could reflect a totally different aspect of cortical folding from cortical surface geometry such as gyrification indices.
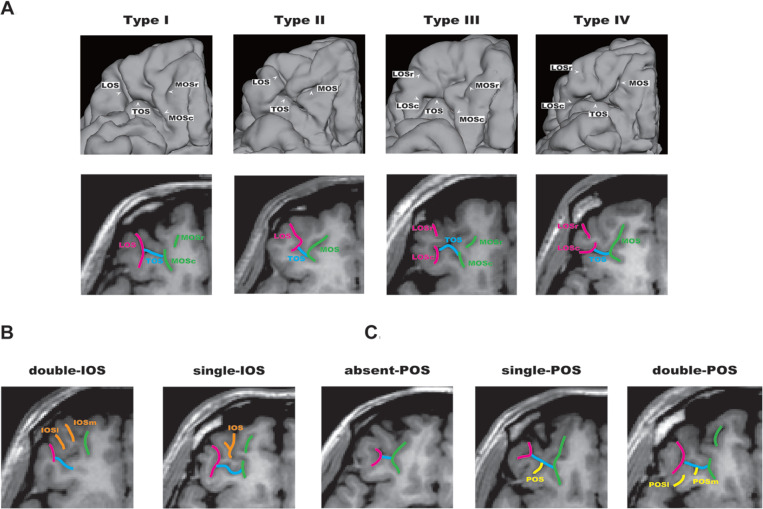
Our group previously reported sulcogyral pattern anomalies in the temporal lobe in schizophrenia using magnetic resonance 3-dimensional surface rendering.16 To investigate the presence of sulcogyral morphological alterations of OFC in schizophrenia, we further focused on the sulcogyral pattern of the “H-shaped” sulcus, which forms the boundary of 4 major orbitofrontal gyri, including medial, anterior, posterior, and lateral orbital gyri.9,17 In the earlier study on OFC comparative neuroanatomy, Chiavaras and Petrides9 classified the OFC sulcogyral pattern into 3 types (type I, type II, type III in order of frequency) in each hemisphere, based on the continuity of the medial and lateral orbital sulci (MOS and LOS, respectively). In type I, rostral and caudal portions of the LOS were connected, while the MOS were clearly interrupted between rostral and caudal portions of MOS. In type II, rostral and caudal portions of both the MOS and LOS were connected, and continuous MOS and LOS were connected by the horizontally oriented transverse orbital sulcus (TOS). In type III, rostral and caudal portions of both MOS and LOS were interrupted. Neighboring orbitofrontal sulci were also identified including the olfactory sulcus (Olf), intermediate orbital sulcus (IOS), posterior orbital sulcus (POS), and sulcus fragmentosus (Fr) (see Figure 1).
Figure 1.
Orbitofrontal cortex (OFC) sulcogyral pattern and its variation in human brain. (A) Patterns of the H-shaped sulcus are classified in 4 subtypes (type I, II, III, IV), according to the continuity of the lateral orbital sulcus (LOS) and medial orbital sulcus (MOS) (-r, rostral; -c, caudal). Type I: continuous LOS and discontinuous MOS. Type II: continuous LOS and MOS. Type III: discontinuous LOS and MOS. Type IV: discontinuous LOS and continuous MOS. (B) Variation of the intermediate orbital sulcus (IOS). The patterns are classified into 2 subtypes depending on the number of identified IOS (-m, medial; -l, lateral). (C) Variation of the posterior orbital sulcus (POS). The patterns are classified into 3 subtypes depending on the number of identified POS (-m, medial; -l, lateral). Panels A, B, and C were adapted and modified from a previous article by Watanabe et al.19 Reprinted with permission of Oxford University Press.
We compared the sulcogyral pattern distribution between schizophrenic patients and matched healthy control subjects. We found that the most common Type I expression was decreased and the least common type III expression was increased in schizophrenia, as compared with healthy controls.8 Furthermore, there was a contrasting association of type III expression with poorer outcome, and that of type I expression with better outcome, suggesting clinical heterogeneity based on early neurodevelopment in schizophrenia. In the data set (n = 100), subjects with type III expression in the right hemisphere showed a 2.84-fold risk for being diagnosed schizophrenia, as compared with subjects without type III expression in the right hemisphere. Following this study, there have been more than 25 studies on OFC sulcogyral morphology reported in several neuropsychiatric disorders. Some of the subsequent studies18 have proposed an additional sulcogyral pattern of type IV, which was the rarest expression (3% to 6 %) in healthy controls, or even an unclassified case in neurodevelopmental disorder.19
As described above, OFC has been regarded as a part of the social brain and associated with several different neuropsychiatric disorders. Also, it has been reported that sulcogyral pattern is formed during early neurodevelopment and stable over time. However, there is yet to be a systematic and integrative review addressing the potential utility of OFC sulcogyral patterns as a potential transdiagnostic trait marker of early neurodevelopment in the social brain. Accordingly, the principal aim guiding this review is to examine and evaluate the current body of empirical evidence bearing on the question of the reliability and validity of abnormalities in OFC sulcogyral patterns as an early neurodevelopmental marker that is shared across different psychiatric disorders or conditions.
Methods
Search Strategy
We conducted an online search of the PubMed/Medline database from the beginning to August 31, 2019. The search string was “orbitofrontal [All Fields] AND sulcogyral [All Fields].” Only English-language articles were included. Studies were excluded if they did not relate to the Chiavaras’s original classification method.9
Data Extraction
The following data were extracted onto a customized table (see Table 1): demographic data (sample size, mean age, gender distribution, diagnosis), methods (magnet, imaging software, voxel size, target sulci, interrater reliability, and statistical strategy), and results regarding the sulcogyral pattern distributions and their group differences and clinical correlations.
Table 1.
Demographic and Clinical Characteristics of Previous Studies.
| Study | Controls Sample Size | Age, y, Mean (SD) | Sex (Male/Female) | Diagnosis | Patients Sample size | Age, y, Mean (SD) | Sex (Male/Female) | Target OFC Sulcus | Other Measures |
|---|---|---|---|---|---|---|---|---|---|
| Chiavaras and Petrides (2000) | 50 | 25.1 (5.3) | 28M/22F | N/A | N/A | N/A | N/A | H-shaped, POS, IOS, etc | |
| Nakamura et al (2007) | 50 | 40.8 (9.4) | 45M/5F | Chronic Sz | 50 | 40.6 (10.4) | 45M/5F | H-shaped | |
| Nakamura et al (2008) | 25 | 41.1 (9.1) | 19M/6F | Chronic Sz | 24 | 39.1 (10.3) | 24M/0F | H-shaped | |
| Chakirova et al (2010) | 36 | 21.2 (2.4) | 17M/19F | High genetic risk of Sz | 146 | 21.2 (2.9) | 74M/72F | H-shaped (+ type IV) | |
| First episode Sz | 34 | 21.4 (3.7) | 23M/11F | ||||||
| Roppongi et al (2010) | 28 | 37.8 (9.8) | 10M/18F | Panic disorder | 28 | 38.4 (9.8) | 10M/18F | POS | |
| Uehara-Aoyama et al (2011) | 47 | 34.7 (10.1) | 17M/30F | Chronic Sz | 47 | 34.2 (10.1) | 23M/24F | H-shaped | |
| Whittle et al (2014) | 152 | 12.6 (0.4) | 80M/72F | N/A | N/A | N/A | N/A | H-shaped | |
| Watanabe et al (2014) | 55 | 32.0 (7.1) | 55M:0F | ASD | 51 | 30.9 (8.2) | 51M:0F | H-shaped, POS, IOS | |
| Bartholomeusz et al (2013) | 73 | 23.7 (5.4) | 48M/25F | First episode Sz | 96 | 21.3 (3.3) | 71M/25F | H-shaped, POS, IOS | LGI, volume |
| Takahashi et al (2014) | 86 | 26.4 (6.6) | 45M/41F | Sz | 72 | 27.5 (6.0) | 39M/33F | H-shaped | YWHAE |
| Lavoie et al (2014) | 58 | 21.4 (3.2) | 37M/21F | UHR transitioned | 49 | 19.5 (3.3) | 29M/19F | H-shaped, POS, IOS | |
| UHR nontransitioned | 77 | 20.6 (3.6) | 44M/32F | ||||||
| Takahashi et al (2015) | 87 | 26.4 (6.6) | 45M/42F | Sz | 75 | 27.4 (6.1) | 41M/34F | H-shaped (+ type IV) | DISC1 |
| Ganella et al (2015) | 147 | 18.1 (0.8) | 62M/85F | EP/ELBW | 194 | 17.9 (0.8) | 87M/107F | H-shaped, POS, IOS | |
| Nishikawa et al (2016) | 84 | 24.5 (5.7) | 47M/37F | Sz | 102 | 25.5 (5.5) | 55M/47F | H-shaped (+ type IV), olfactory sulcus | |
| Schizotypal (SPD) | 47 | 25.0 (5.4) | 29M/18F | ||||||
| Cropley et al (2015) | 87 | 26.9 (10.0) | 55M/32F | Chronic Sz | 89 | 34.9 (9.6) | 76M/13F | H-shaped | |
| Yoshimi et al (2016) | 60 | 33.6 (10.3) | 29M/31F | Sz | 59 | 31.4 (9.7) | 23M/36F | H-shaped | NRG1 |
| Takahashi et al (2016) | 84 | 24.5 (5.7) | 47M/37F | Sz | 102 | 25.5 (5.5) | 55M/47F | POS, IOS | |
| Schizotypal (SPD) | 47 | 25.0 (5.4) | 29M/18F | ||||||
| Zhang et al (2016) | 58 | 23.3 (4.0) | 28M/30F | N/A | N/A | N/A | N/A | H-shaped | |
| Takahashi et al (2017) | 59 | 26.1(5.1) | 28M/31F | Deficit Sz | 38 | 27.1(6.2) | 22M/16F | H-shaped, POS, IOS | AI, CSP |
| Nondeficit Sz | 37 | 27.1(7.5) | 12M/25F | ||||||
| Isomura et al (2017) | 375 | 36.5 (12.8) | 185M/190F | Sz | 155 | 36.0 (11.0) | 94M/61F | H-shaped | |
| Chye et al (2017) | 128 | 26.3 (8.9) | 91M/37F | CB user | 146 | 28.4 (10.5) | 98M/48F | H-shaped | |
| Patti et al (2017) | 53 | 31.1 (9.1) | 24M/29F | Sz | 49 | 36.5 (9.0) | 37M/12F | H-shaped | |
| Bipolar disorder | 46 | 35.7 (9.3) | 27M/19F | ||||||
| ADHD | 41 | 32.7 (10.6) | 21M/20F | ||||||
| Nakamura et al (2018) | 110 | 21.3 (3.2) | 54M/56F | ARMS | 125 | 21.3(5.5) | 54M/71F | H-shaped, POS, IOS | |
| Delahoy et al (2019) | 78 | 33.1 (10.1) | 42M/36F | OCD | 80 | 33.0 (8.3) | 42M/38F | H-shaped | |
| Takahashi et al (2019) | 61 | 25.6 (3.2) | 32M/29F | ARMS | 38 | 18.4 (3.9) | 24M/14F | H-shaped, POS, IOS | |
| Sz | 63 | 28.0 (9.4) | 29M/34F | ||||||
| Li et al. (2019) | 159 | 33.0 (9.8) | 154M/5F | PG | 165 | 34.3 (10.0) | 164M/1F | H-shaped (+ type IV) |
Abbreviations: SD, standard deviation; Sz, schizophrenia; ASD, autism spectrum disorder; UHR, ultra high risk; EP/ELBW, extremely preterm/extremely low birth weight; SPD, schizotypal personality disorder; CB, cannabis; ADHD, attention-deficit/hyperactivity disorder; ARMS, at-risk mental state; OCD, obsessive-compulsive disorder; PG, pathological gambling; POS, posterior orbital sulcus; IOS, intermediate orbital sulcus; GI, gyrification index; AI, adhesio interthalamica; CSP, cavum septi pellucidi; N/A, data not applicable.
Results
A total of 26 articles were identified. After screening titles and abstracts, 24 articles met eligibility criteria. One article was excluded due to not being in English20 and another article was excluded due it being a methodology paper.21 Chiavaras and Petrides’s original article9 and a single recent article22 were added by quotations within quotations. Demographics and methods for the 26 studies are summarized in Table 1.
Out of the 26 studies, 16 (62%) focused on schizophrenia or schizophrenia spectrum, including schizotypal personality disorder (SPD; n = 2), and ultra high risk (UHR; n = 2), and at-risk mental state (ARMS; n = 2) population for psychosis. Three studies focused on neurodevelopmental disorders or states of autism spectrum disorder (ASD; n = 1) and attention-deficit/hyperactivity disorder (ADHD; n = 1) or developmental history of extremely preterm (<28 weeks) and extremely low birth weight (<1000 g) (EP/ELBW; n = 1). Only 3 studies focused on healthy volunteers. The remaining studies focused on panic disorder, obsessive-compulsive disorder (OCD; n = 1), bipolar disorder (BD; n = 1), cannabis users (CB; n = 1), and pathological gambling (PG; n = 1).
As shown in Table 1, 24 out of 26 studies investigated the “H-shaped” sulcus, 10 of 26 studies investigated POS, 9 of 26 studies investigated IOS, and 2 of 26 studies investigated olfactory sulcus (Olf), with some overlaps. Also, 3 out of 26 studies investigated the association between OFC sulcogyral pattern and gene polymorphisms (YWHAE, DISC 1, and NRG 1) in order to clarify genetic contribution to the sulcogyral pattern.
In terms of methodology, 17 (65.4 %) studies were performed on 1.5-T magnetic resonance imaging (MRI) scanners, while 5 (19.2%) were performed on 3.0-T MRI scanners and 3 (11.5%) were based on mixed data from 1.5- and 3.0-T MRI scanners and a single study18 was performed on 1.0-T MRI scanner. Voxel size was mostly 1.0 mm isovoxel or 0.9375 × 0.9375 × 1.5 mm. Imaging software used included 3D slicer (https://www.slicer.org), Dr. View (Infocom, Tokyo, Japan), Analyze (Mayo Clinic), FSL (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki), and MRIcro (https://people.cas.sc.edu/rorden/mricro/index.html). Interrater reliability (Cronbach’s alpha) of the sulcogyral pattern was generally more than .8. For statistical strategy, a chi-square test was used for group comparisons and categorical regression or 1-way analysis of variance models were employed for correlational analysis.
Results (group difference and correlations) are summarized in Table 2.
Table 2.
Summary Table of Major Findings of Previous Studies.
| Study | Diagnosis | Results (Group Difference) | Results (Correlation) |
|---|---|---|---|
| Chiavaras and Petrides (2000) | N/A | Type I was most common and type III was least common variant (HC). | |
| Nakamura et al (2007) | Chronic Sz | Sz showed increased type III and decreased type I in right OFC dominant. | Type III was associated with poorer cognitive function, more severe symptom, impulsivity and small ICC. |
| Nakamura et al (2008) | Chronic Sz | OFC volume was not associated with OFC sulcogyral pattern | |
| Chakirova et al (2010) | High genetic risk of Sz | Difference between high-risk transitioned and nontransitioned (decreased type I and increased type III). | Type III was associated with higher SIS score in high risk of Sz. |
| First episode Sz | Difference between FESz and HC (decreased type I and increased type III). | ||
| Roppongi et al (2010) | Panic disorder | No difference | Absent or single POS was associated with smaller volume of right posterior-medial OFC in PD. |
| Uehara-Aoyama et al (2011) | Chronic Sz | Male Sz showed increased type III but not in female Sz. | Type III was associated with higher PANSS score in male Sz. |
| Whittle et al (2014) | N/A | N/A | Type I was associated with low level of surgency, high level of negative affectivity and depressive symptoms. |
| Watanabe et al (2014) | ASD | ASD showed increased type III in bilateral OFC. Fewer POS in ASD. | Type I was associated with reduced AQ total score and its subscale of “attention to detail.” |
| Bartholomeusz et al (2013) | First episode Sz | In right OFC, Sz showed decreased type I and increased type II. Fewer IOS in left OFC of Sz. | No relationship between H-shaped sulcus pattern and OFC gyrification index. |
| Takahashi et al (2014) | Sz | Protective C allele carriers (YWHAE) showed decreased type III and increased type I in left OFC of HC. | |
| Lavoie et al (2014) | UHR transitioned | UHR transitioned showed reduced type I in right OFC. UHR transitioned showed fewer IOS and POS. | |
| UHR nontransitioned | |||
| Takahashi et al (2015) | Sz | Cys carriers (DISC1) showed reduced type I in right OFC of HC (but not SZ). | |
| Ganella et al (2015) | EP/ELBW | In left OFC, EP/ELBW showed increased type II and fewer IOS and increased POS. | Type III was associated with better metacognition sores and behavioral regulation scores. |
| Nishikawa et al (2016) | Sz | Sz showed increased type III and decreased type I. SPD did not differ from HC. Sz and SPD showed shallower olfactory sulcus. | |
| Schizotypal (SPD) | |||
| Cropley et al (2015) | Chronic Sz | Sz showed increased type II. | |
| Yoshimi et al (2016) | Sz | Sz showed increased type III. | Type III was associated with earlier age of onset. NRG1 SNP was associated with type II expression. |
| Takahashi et al (2016) | Sz | Sz and SPD showed fewer number of IOS and POS. | Fewer sulci were association with negative symptom |
| Schizotypal (SPD) | |||
| Zhang et al (2016) | N/A | N/A | Physical anhedonia was associated with low incidence of type I and high incidence of type II in left OFC. |
| Takahashi et al (2017) | Deficit Sz | Deficit Sz showed decreased type I, increased type III in right OFC, and fewer POS as compared with HC. | |
| Nondeficit Sz | |||
| Isomura et al (2017) | Sz | Female Sz showed decreased type I and increased type II. | |
| Chye et al (2017) | CB user | No difference | Type III in the right OFC tended to use more CB over lifetime. |
| Patti et al (2017) | Sz | Sz and BP showed increased type III/IV and reduced type I in left OFC. | |
| Bipolar disorder | |||
| ADHD | ADHD group showed a trend-level difference from HC. | ||
| Nakamura et al (2018) | ARMS | ARMS as a whole had fewer number of IOS and POS. No difference in H-shaped sulci. | Fewer number of IOS and POS was associated with prodoromal symptomatology. |
| Delahoy et al (2019) | OCD | No difference | Type II and type III was associated with OCD severity. |
| Takahashi et al (2019) | ARMS | Both ARMS and Sz showed increased type III in right OFC and fewer IOS and POS. | association with social and cognitive impairment. |
| Sz | |||
| Li et al (2019) | PG | PG showed increased type II in bilateral OFC. | No clinical correlation with PG severity. |
Abbreviations: SD, standard deviation; Sz, schizophrenia; ASD, autism spectrum disorder; UHR, ultra high risk; EP/ELBW, extremely preterm/extremely low birth weight; SPD, schizotypal personality disorder; CB, cannabis; ADHD, attention-deficit/hyperactivity disorder; ARMS, at-risk mental state; OCD, obsessive-compulsive disorder; PG, pathological gambling; POS, posterior orbital sulcus; IOS, intermediate orbital sulcus; LGI, local gyrification index; SIS, Structured Inventory for Schizotypy; SNP, single nucleotide polymorphism; PANSS, Positive and Negative Syndrome Scale; AQ, autism spectrum quotient; N/A, data not applicable.
Schizophrenia Spectrum
In schizophrenia spectrum, OFC sulcogyral pattern has been investigated in chronic schizophrenia (CSz), first episode schizophrenia (FESz), SPD, and UHR/ARMS for psychosis. In CSz, incidence of type I expression8,23,24 was decreased and those of type III8,23,25,26 and type II24,27 expressions were increased as compared with healthy controls. In terms of hemispheric laterality or asymmetry, some studies8,18,22,26,28,29 reported right-dominant alteration of the OFC sulcogyral pattern distribution in CSz, while other studies23,30 reported left-dominant alteration. Sex contribution of this alteration has, however, been inconsistent. One study26 reported a male-specific increased incidence of type III expression in CSz, while another study24 reported a female-specific alteration of decreased type I and increased type II expressions. Also, OFC sulcogyral pattern was not associated with OFC gray matter volume,31 while type III expression was associated with smaller intracranial contents,8 which may reflect an early neurodevelopmental process. Of note, type III expression was associated with more severe symptoms, poorer cognitive function, impulsivity, and earlier age of onset.8,25 In terms of a genetic contribution to the sulcogyral pattern, a NRG1 single nucleotide polymorphism (SNP), which could relate to myelination and neuronal migration, was associated with type II expression.25 More specifically, CSz patients who were homozygotes with the minor allele of SNP8NRG243177 showed a decreased incidence of type II expression as compared with heterozygotes or major homozygotes.
In studies of FESz, SPD, and UHR/ARMS for psychosis, both FESz and UHR/ARMS subjects who transitioned to psychosis showed decreased incidence of type I expression and increased incidence of type III and type II expressions,18,22,29,30,32 while SPD and UHR/ARMS subjects who did not transition to psychosis did not show such alteration. Moreover, decreased numbers of IOS and POS were consistently reported in FESz, UHR/ARMS (transitioned as well as nontransitioned), and SPD subjects.22,29,30,33,34 Additionally, FESz and SPD showed shallower Olf as compared with healthy controls.32 Hemispheric laterality of findings for OFC sulcogyral patterns has been inconsistent and sex dimorphism in the sulcogyral pattern distribution has not been reported in these populations. The gyrification index was also not associated with OFC sulcogyral patterns.30 Of note, type III expression in UHR/ARMS was associated with higher scores on the psychotic factor of the Structured Inventory for Schizotypy18 and poorer social functioning.22 Fewer number of IOS and POS was also associated with prodromal symptomatology,33 and poorer cognitive function.22 In terms of genetic contribution, neurodevelopment-related SNPs of YWHAE (rs28365859) and DISC1 (Ser704Cys) have been reported to be associated with the OFC sulcogyral pattern in healthy controls but not in FESz groups. More specifically, healthy subjects with the protective C allele (YWHAE) were shown to exhibit increased incidence of type I expression and decreased incidence of type III expression as compared with the G allele homozygotes.35 Also, healthy subjects with the risk-related Cys allele (DISC1) showed decreased incidence of type I expression.36
In summary, sulcogyral pattern distribution of the H-shaped sulci has been reported to be altered (decreased incidence of type I and increased incidence of type III or II) in both CSz and FESz, and partly in UHR/ARMS transitioned but not in SPD or in UHR/ARMS nontransitioned. Fewer numbers of IOS and POS have been reported in FESz, SPD, and UHR/ARMS as a whole. Additionally, dominance in hemispheric laterality and sex dimorphism have thus far not been consistently reported. Neither OFC gray matter volume nor OFC gyrification index have been shown to be correlated with OFC sulcogyral patterns. Type III expression of H-shaped sulci has been clinically associated with more severe symptoms, higher psychotic factor on the Structured Inventory for Schizotypy, poorer cognitive functioning, poorer social functioning, and earlier age of onset. Fewer numbers of IOS and POS in schizophrenia spectrum have been reported to be associated with prodromal symptomatology and poorer cognitive functioning. Genetic association with the OFC sulcogyral pattern has not been as straightforward, although healthy controls show a different pattern than what is observed in the patient population. Furthermore, more work is needed to investigate further the possible protective effects of the C allele (YWHAE) and the risk-related Cys allele (DISC1).
Other Disorders and Healthy Population
A single study investigated the OFC sulcogyral pattern in ASD. More specifically, Watanabe et al19 reported that adult male ASD subjects show increased incidence of type III expression in bilateral OFC, and a fewer number of POS in right OFC. Type I expression was also associated with a milder score in the “attention to detail” subscale of Autism Spectrum Quotient (AQ). Of note, it has been reported that a single ASD case with high IQ was not able to be classified into any known type of H-shaped sulci, with no visible IOS, unclear TOS, and unclear caudal parts of MOS and LOS.
Patti et al23 reported a transdiagnostic comparison among CSz, BD, and ADHD. These authors also reported that both CSz and BD groups exhibited similar atypical distributions (increased type III/IV and reduced type I expressions) in the left OFC. The ADHD group showed a marginally significant alteration as compared with healthy controls, with trend-level differences (P = .063 in left OFC and P = .053 in right OFC), similar to the alterations observed in CSz and BD groups. Birth history of EP/ELBW were associated with increased type II expression and fewer number of IOS and increased number of POS in left OFC.37 Additionally, the Behavior Rating Inventory of Executive Function Questionnaire showed that type III expression was associated with better parent-reported metacognition scores across healthy control and EP/ELBW groups, and with better self-reported behavioral regulation scores in the control group of adolescents.
Of note here, although there have been only a small number of studies of BD and neurodevelopmental disorders such as ASD and ADHD, both disorders have shown a similar alteration of OFC sulcogyral pattern to that observed in the schizophrenia spectrum disorder, possibly reflecting transdiagnostic aspects of the early neurodevelopment of the social brain.
In terms of addictive disorders, CB users have not been shown to differ in the OFC sulcogyral pattern from healthy controls, while CB users with type III in the right OFC have tended to use more CB over their lifetime.38 Patients with a gambling disorder have shown increased type II expression in both hemispheres as compared with healthy controls.39 Addictive disorders linked to decision-making and reward processing may therefore be associated, in part, with early neurodevelopment of the OFC and a possible premorbid trait marker.
The OFC sulcogyral pattern distribution was not, however, observed to be altered in panic disorder40 or OCD,41 although type II and III expressions were correlated with OCD severity. In previous studies focused on healthy populations using a revised version the Early Adolescent Temperament Questionnaire, type I expression in adolescents was associated with low levels of surgency and high levels of negative affectivity and depressive symptoms,42 presumably reflecting temperamental risk for psychopathology. Another study focused on hedonic experience and reported that physical anhedonia was associated with decreased type I and increased type II expression in the left OFC of healthy young adults.43
Discussion
The present review of the OFC sulcogyral pattern shows that type I expression may be a neurodevelopmental protective marker whereas type II and III expression may be neurodevelopmental risk markers. Also, fewer numbers of IOS and POS might also reflect a neurodevelopmental risk marker. It should be noted here, however, that the increased risk markers and decreased protective markers of the OFC sulcogyral pattern have been consistently reported in the schizophrenia spectrum, although the specificity in sex and hemispheric dominance have not been consistently reported across studies. Furthermore, the risk markers of type III expression were associated with more severe symptoms and poorer cognitive and social functioning.8,18,25,26
According to the McCarley’s 2-hit model of schizophrenia,44 the “first hit” occurs during neurodevelopment resulting in alterations of the sulcogyral pattern, ventricular system, and brain asymmetry or torque. The current review strongly supports McCarley’s model in terms of early neurodevelopment of OFC as a social brain in schizophrenia spectrum. One might argue that the “second hit” of schizophrenia such as progressive deterioration in prefrontal structures or any secondary effects of the illness could account for the altered OFC sulcogyral pattern. However, we think this unlikely because the sulcogyral pattern is set in early neurodevelopment and is independent of brain tissue volume changes following neurodevelopment. Thus, the OFC sulcogyral pattern alterations observed can be viewed as susceptibility or vulnerability marker as opposed to a diagnostic marker. On an individual basis, the OFC sulcogyral pattern may account for neurodevelopmental aspects of the clinical heterogeneity of schizophrenia, serving as a modulatory marker. A patient with the OFC risk factors may show an earlier onset, more severe symptoms, and be more treatment resistant.8
Although McCarley did not refer to disease specificity of the first hit, alterations of the OFC sulcogyral pattern may be transdiagnostic, especially across BD and neurodevelopmental disorders such as ASD and ADHD. Furthermore, while there have only been a small number of studies, OCD and addictive disorder of CB users have not shown alterations of the OFC sulcogyral pattern. However, the OFC sulcogyral risk factors such as type III were associated with disease severity in these clinical populations of OCD and addiction was strongly linked to OFC pathophysiological involvement.
It is also of interest that the OFC sulcogyral pattern was associated with hedonic experience even in healthy population.43 However, one important caveat is that 2 studies of healthy adolescents have reported the presumed protective type I expression to be associated with higher level of negative affectivity and depressive symptoms,42 and the presumed risk-increasing type III to be associated with better self-reported behavioral regulation scores.37 More studies of neurodevelopmental disorders as well as healthy populations are nonetheless needed so that we can utilize the OFC sulcogyral pattern as a transdiagnostic trait marker of social functioning, including emotional processing, reward evaluation, motivation, goal-directed behavior, and so on.
In terms of methodological consideration, interrater reliability for the OFC sulcogyral pattern classification has been reported as high, and mostly greater than .8. However, such a visual classification method could yield some inconsistencies especially for intermediate patterns between type I and III or between Type I and II. Also, the rarest type IV expression, proposed by Chakirova et al,18 may be included in such ambiguous patterns. Empirically, such ambiguous patterns seem to be observed in 20% to 30% of whole hemispheres. Of note here, there have been a few studies30,41 reporting healthy control data of H-shaped sulcus pattern distributions, which were quite different from Chiavaras and Petrides’s original report. In these reports, frequency of type III expression was more often than that of type II expression in healthy controls. Similarly, in clinical populations such as schizophrenia spectrum disorders, some studies reported increased incidence of type III expression8,18,22,23,25,26,28,32 and others24,27,30 reported increased incidence of type II expression as compared with healthy controls. Although these discrepancies between type II and III expressions may be attributable to sampling bias or classification methodology, further investigations are needed to understand them more clearly.
Recently, automated tracing methods of the OFC sulci employing the BrainVISA Morphologist Pipeline have been proposed.21 This kind of approach could make the OFC sulcogyral classification less arbitrary and time-consuming, thereby making it possible to analyze a large population with high reliability. Moreover, the sulcogyral pattern is likely independent of cortical volumes and gyrification indices. Therefore, this unique categorical measure of sulcogyral pattern may be less susceptible to biases of MRI scanners, MRI vendors, protocols, different software for postprocessing, and so on. Given this likely advantage of the OCF sulcogyral pattern, it could be measured in a more automated fashion and extended to a large data set from multicenter studies.
In conclusion, based on the current review of the literature, we view the OFC region, a crucial part of the social brain, as likely involved in many neuropsychiatric disorders, including, in particular, schizophrenia, bipolar disorder, obsessive-compulsive disorder, and in a broad range of addiction. The OFC sulcogyral pattern should be investigated further as a common modulator of social functioning in these different clinical entities.
Acknowledgments
The authors gratefully acknowledge the administrative support of Marie Fairbanks and the great mentoring of past Prof Robert W. McCarley.
Footnotes
Full-color figures are available online at journals.sagepub.com/home/eeg
Author Contributions: MN contributed to conception and design; contributed to acquisition, analysis, and interpretation; drafted manuscript; critically revised manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy. PGN contributed to conception and design; contributed to interpretation; critically revised manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy. MES contributed to conception and design; contributed to interpretation; critically revised manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by AMED under grant numbers JP19dm0307001 (MN) and JP19dm0307105(MN), and a VA Merit Award (MES).
ORCID iDs: Motoaki Nakamura  https://orcid.org/0000-0003-2830-3049
https://orcid.org/0000-0003-2830-3049
Paul G. Nestor  https://orcid.org/0000-0003-4890-8950
https://orcid.org/0000-0003-4890-8950
References
- 1. Harlow JM. Passage of an iron rod through the head. Boston Med Surg J. 1848;39:389-393. [PMC free article] [PubMed] [Google Scholar]
- 2. Bleuler E. Dementia Praecox of the Group of Schizophrenias (1911). Zinkin J, trans. New York, NY: International Universities Press; 1950. [Google Scholar]
- 3. Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206-219. [DOI] [PubMed] [Google Scholar]
- 4. Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci. 2005;6:691-702. [DOI] [PubMed] [Google Scholar]
- 5. Walton ME, Devlin JT, Rushworth MF. Interactions between decision making and performance monitoring within prefrontal cortex. Nat Neurosci. 2004;7:1259-1265. [DOI] [PubMed] [Google Scholar]
- 6. Holland PC, Gallagher M. Amygdala-frontal interactions and reward expectancy. Curr Opin Neurobiol. 2004;14:148-155. [DOI] [PubMed] [Google Scholar]
- 7. Gottfried JA, O’Doherty J, Dolan RJ. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science. 2003;301:1104-1107. [DOI] [PubMed] [Google Scholar]
- 8. Nakamura M, Nestor PG, McCarley RW, et al. Altered orbitofrontal sulcogyral pattern in schizophrenia. Brain. 2007;130(pt 3):693-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chiavaras MM, Petrides M. Orbitofrontal sulci of the human and macaque monkey brain. J Comp Neurol. 2000;422:35-54. [PubMed] [Google Scholar]
- 10. Ono M, Kubik S, Abernathey CD. Atlas of the Cerebral Sulci. New York, NY: Thieme; 1990. [Google Scholar]
- 11. Armstrong E, Schleicher A, Omran H, Curtis M, Zilles K. The ontogeny of human gyrification. Cereb Cortex. 1995;5:56-63. [DOI] [PubMed] [Google Scholar]
- 12. Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170-176. [DOI] [PubMed] [Google Scholar]
- 13. Zilles K, Armstrong E, Schleicher A, Kretschmann HJ. The human pattern of gyrification in the cerebral cortex. Anat Embryol (Berl). 1988;179:173-179. [DOI] [PubMed] [Google Scholar]
- 14. Schaer M, Cuadra MB, Tamarit L, Lazeyras F, Eliez S, Thiran JP. A surface-based approach to quantify local cortical gyrification. IEEE Trans Med Imaging. 2008;27:161-170. [DOI] [PubMed] [Google Scholar]
- 15. Bartley AJ, Jones DW, Weinberger DR. Genetic variability of human brain size and cortical gyral patterns. Brain. 1997;120(pt 2):257-269. [DOI] [PubMed] [Google Scholar]
- 16. Kikinis R, Shenton ME, Gerig G, et al. Temporal lobe sulco-gyral pattern anomalies in schizophrenia: an in vivo MR three-dimensional surface rendering study. Neurosci Lett. 1994;182:7-12. [DOI] [PubMed] [Google Scholar]
- 17. Duvernoy HM. The Human Brain: Surface, Three-Dimensional Sectional Anatomy With MRI, and Blood Supply. 2nd ed. New York, NY: Springer-Verlag Wien; 1999. [Google Scholar]
- 18. Chakirova G, Welch KA, Moorhead TW, et al. Orbitofrontal morphology in people at high risk of developing schizophrenia. Eur Psychiatry. 2010;25:366-372. [DOI] [PubMed] [Google Scholar]
- 19. Watanabe H, Nakamura M, Ohno T, et al. Altered orbitofrontal sulcogyral patterns in adult males with high-functioning autism spectrum disorders. Soc Cogn Affect Neurosci. 2014;9:520-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Roppongi T, Hirayasu Y. Association of the posterior orbitofrontal sulcogyral pattern with orbitofrontal cortex volume reduction and anxiety trait in panic disorder [in Japanese]. Seishin Shinkeigaku Zasshi. 2013;115:357-362. [PubMed] [Google Scholar]
- 21. Snyder W, Patti M, Troiani V. An evaluation of automated tracing for orbitofrontal cortex sulcogyral pattern typing. J Neurosci Methods. 2019;326:108386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Takahashi T, Nakamura M, Nishikawa Y, et al. Potential role of orbitofrontal surface morphology on social and cognitive functions in high-risk subjects for psychosis and schizophrenia patients. Psychiatry Res Neuroimaging. 2019;283:92-95. [DOI] [PubMed] [Google Scholar]
- 23. Patti MA, Troiani V. Orbitofrontal sulcogyral morphology is a transdiagnostic indicator of brain dysfunction. Neuroimage Clin. 2018;17:910-917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Isomura S, Hashimoto R, Nakamura M, et al. Altered sulcogyral patterns of orbitofrontal cortex in a large cohort of patients with schizophrenia. NPJ Schizophr. 2017;3:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yoshimi A, Suda A, Hayano F, et al. Effects of NRG1 genotypes on orbitofrontal sulcogyral patterns in Japanese patients diagnosed with schizophrenia. Psychiatry Clin Neurosci. 2016;70:261-268. [DOI] [PubMed] [Google Scholar]
- 26. Uehara-Aoyama K, Nakamura M, Asami T, et al. Sexually dimorphic distribution of orbitofrontal sulcogyral pattern in schizophrenia. Psychiatry Clin Neurosci. 2011;65:483-489. [DOI] [PubMed] [Google Scholar]
- 27. Cropley VL, Bartholomeusz CF, Wu P, et al. Investigation of orbitofrontal sulcogyral pattern in chronic schizophrenia. Psychiatry Res. 2015;234:280-283. [DOI] [PubMed] [Google Scholar]
- 28. Takahashi T, Takayanagi Y, Nishikawa Y, et al. Brain neurodevelopmental markers related to the deficit subtype of schizophrenia. Psychiatry Res Neuroimaging. 2017;266:10-18. [DOI] [PubMed] [Google Scholar]
- 29. Lavoie S, Bartholomeuz CF, Nelson B, et al. Sulcogyral pattern and sulcal count of the orbitofrontal cortex in individuals at ultra high risk for psychosis. Schizophr Res. 2014;154:93-99. [DOI] [PubMed] [Google Scholar]
- 30. Bartholomeusz CF, Whittle SL, Montague A, et al. Sulcogyral patterns and morphological abnormalities of the orbitofrontal cortex in psychosis. Prog Neuropsychopharmacol Biol Psychiatry. 2013;44:168-177. [DOI] [PubMed] [Google Scholar]
- 31. Nakamura M, Nestor PG, Levitt JJ, et al. Orbitofrontal volume deficit in schizophrenia and thought disorder. Brain. 2008;131(pt 1):180-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nishikawa Y, Takahashi T, Takayanagi Y, et al. Orbitofrontal sulcogyral pattern and olfactory sulcus depth in the schizophrenia spectrum. Eur Arch Psychiatry Clin Neurosci. 2016;266:15-23. [DOI] [PubMed] [Google Scholar]
- 33. Nakamura M, Takahashi T, Takayanagi Y, et al. Surface morphology of the orbitofrontal cortex in individuals at risk of psychosis: a multicenter study. Eur Arch Psychiatry Clin Neurosci. 2019;269:397-406. [DOI] [PubMed] [Google Scholar]
- 34. Takahashi T, Nakamura M, Nishikawa Y, et al. Decreased number of orbital sulci in schizophrenia spectrum disorders. Psychiatry Res Neuroimaging. 2016;250:29-32. [DOI] [PubMed] [Google Scholar]
- 35. Takahashi T, Nakamura Y, Nakamura Y, et al. The polymorphism of YWHAE, a gene encoding 14-3-3epsilon, and orbitofrontal sulcogyral pattern in patients with schizophrenia and healthy subjects. Prog Neuropsychopharmacol Biol Psychiatry. 2014;51:166-171. [DOI] [PubMed] [Google Scholar]
- 36. Takahashi T, Nakamura M, Nakamura Y, et al. The disrupted-in-schizophrenia-1 Ser704Cys polymorphism and brain neurodevelopmental markers in schizophrenia and healthy subjects. Prog Neuropsychopharmacol Biol Psychiatry. 2015;56:11-17. [DOI] [PubMed] [Google Scholar]
- 37. Ganella EP, Burnett A, Cheong J, et al. ; Victorian Infant Collaborative Study Group. Abnormalities in orbitofrontal cortex gyrification and mental health outcomes in adolescents born extremely preterm and/or at an extremely low birth weight. Hum Brain Mapp. 2015;36:1138-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chye Y, Solowij N, Ganella EP, et al. Role of orbitofrontal sulcogyral pattern on lifetime cannabis use and depressive symptoms. Prog Neuropsychopharmacol Biol Psychiatry. 2017;79(pt B):392-400. [DOI] [PubMed] [Google Scholar]
- 39. Li Y, Wang Z, Boileau I, et al. Altered orbitofrontal sulcogyral patterns in gambling disorder: a multicenter study. Transl Psychiatry. 2019;9:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Roppongi T, Nakamura M, Asami T, et al. Posterior orbitofrontal sulcogyral pattern associated with orbitofrontal cortex volume reduction and anxiety trait in panic disorder. Psychiatry Clin Neurosci. 2010;64:318-326. [DOI] [PubMed] [Google Scholar]
- 41. Delahoy R, Bartholomeusz CF, Pemberton H, et al. An examination of orbitofrontal sulcogyral morphology in obsessive-compulsive disorder. Psychiatry Res Neuroimaging. 2019;286:18-23. [DOI] [PubMed] [Google Scholar]
- 42. Whittle S, Bartholomeusz C, Yucel M, Dennison M, Vijayakumar N, Allen NB. Orbitofrontal sulcogyral patterns are related to temperamental risk for psychopathology. Soc Cogn Affect Neurosci. 2014;9:232-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang H, Harris L, Split M, Troiani V, Olson IR. Anhedonia and individual differences in orbitofrontal cortex sulcogyral morphology. Hum Brain Mapp. 2016;37:3873-3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McCarley RW, Wible CG, Frumin M, et al. MRI anatomy of schizophrenia. Biol Psychiatry. 1999;45:1099-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]