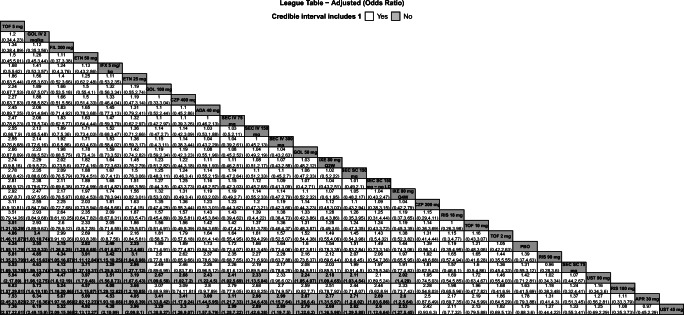
Fig. 2.
League table of pairwise comparisons for all treatments in the ASAS20 NMA. Pairwise comparisons are reported as ORs and 95% Crls. Comparators are ordered from largest (top-left) to smallest (bottom-right) SUCRA values for the ASAS20 NMA. Please refer to Fig. 5 for SUCRA values for each NMA. Superior improvements in ASAS20 are denoted in bold text and light gray cells. Results are shown for the baseline risk-adjusted model for ASAS20. Please refer to Appendix S6 for the model fit statistics. Abbreviations: ADA, adalimumab; APR, apremilast; ASAS20, improvement of ≥ 20% in the Assessment of Spondyloarthritis International Society Criteria; Crls, credible intervals; CZP, certolizumab pegol; ETN, etanercept; FIL, filgotinib; GOL, golimumab; IFX, infliximab; IXE, ixekizumab; IV, intravenous; LD, loading dose; NMA, network meta-analysis; OR, odds ratio; PBO, placebo; Q2W, every 2 weeks; Q4W, every 4 weeks; RIS, risankizumab; SC, subcutaneous; SEC, secukinumab; SUCRA, Surface Under the Cumulative Ranking curve; TOF, tofacitinib; UST, ustekinumab