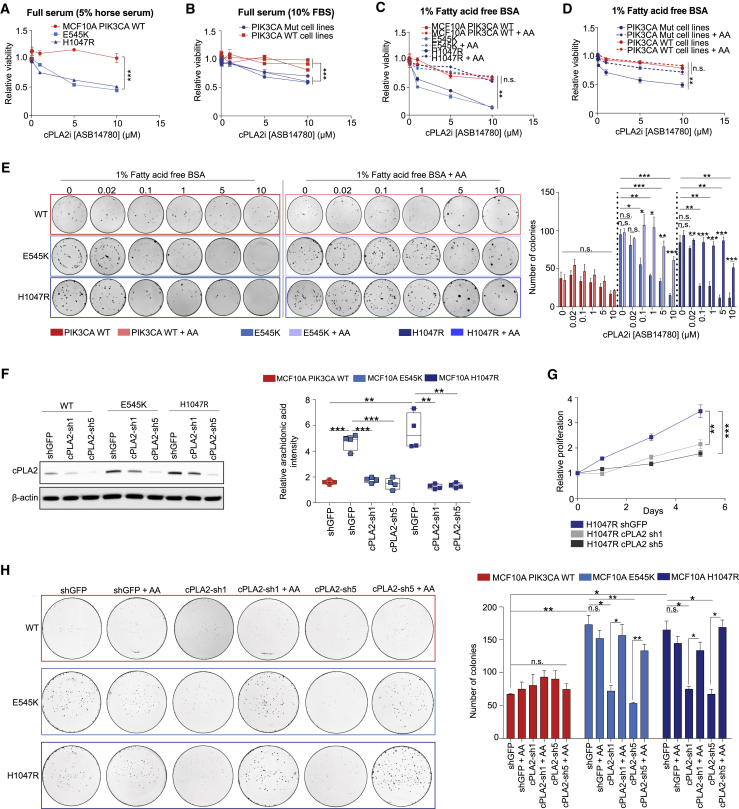
Figure 5.
Genetic and Pharmacological Inhibition of cPLA2 Selectively Reduces Oncogenic PIK3CA-Mediated Tumorigenicity
(A–D) Cell viability of (A) PIK3CA WT and MUT MCF10A cells, and (B) breast cancer cell lines (PIK3CA WT: MDAMB134, Hs578T, AU565; PIK3CA MUT: MCF-7, CAL-51, MDAMB453) following treatment with increasing concentrations (20 nM–10 μM) of ASB14780 under full serum conditions for 72 h. The same treatments were also performed under fatty acid-free conditions in (C) and (D), in the presence or absence of exogenous supplementation of 25 μM AA.
(E) Clonogenic assays of MCF10A PIK3CA WT and MUT cells treated with increasing concentrations of ASB14780 as in (A)–(D). Treatments were performed under fatty acid-free conditions, with or without the supplementation of 25 μM AA.
(F) Immunoblot analysis confirming specific knockdown of cPLA2 using two independent constitutive shRNAs (sh1 and sh5) (left) and reduction in AA levels in MCF10A E545K/H1047R MUT cells using REIMS.
(G) Proliferation of MCF10A H1047R MUT cells expressing shGFP, cPLA2-sh1, or cPLA2-sh5 under exogenous FAF conditions. Sulforhodamine B (SRB) protein staining was used to measure cell proliferation over 5 days.
(H) Clonogenic assays of MCF10A PIK3CA WT and MUT cells expressing shGFP, cPLA2-sh1, or cPLA2-sh5 under FAF conditions, supplemented with or without 25 μM AA. Data in (A)–(H) are presented as the mean ± SEM of n = 3–4 biological replicates and are representative of at least two independent experiments. Data in (D) are presented as the mean viability of three PIK3CA MUT (MCF-7, CAL-51, MDAMB453) and WT (MDAMB134, Hs578T, AU565) measured in triplicate wells. n.s., not significant; ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; p values in (A)–(D) and (G) were calculated using two-way ANOVA. For (E, right), (F, right), and (H, right), one-way ANOVA followed by unpaired, two-tailed Student’s t test with Bonferroni correction was applied.