FIGURE 4.
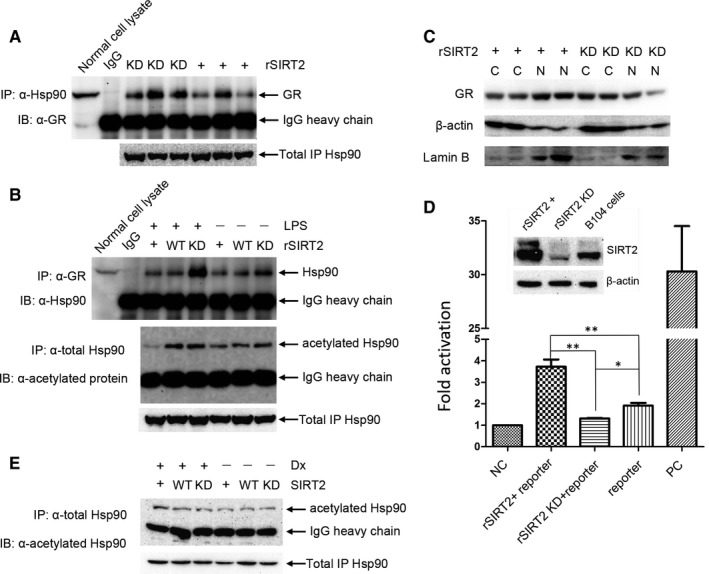
Co‐IP assays was carried out with anti‐Hsp90 in B104 cells in which SIRT2 was overexpression or knocked‐down, and glucocorticoid receptor (GR) was detected in the IP complex by immunoblot (A). SIRT2 overexpression resulted in decreased interaction between Hsp90 and GR compared with SIRT2 knock‐down. Co‐IP assays were also performed with anti‐GR in SIRT2 overexpression, SIRT2 knocked‐down or control cells, and Hsp90 was detected in the IP complex by immunoblot (B; top panel). Co‐IP with anti‐Hsp90 under the same conditions, followed by detection of acetylated Hsp90 (B; bottom panel), showed that the interaction between Hsp90 and GR corresponds to the acetylation levels of Hsp90. (C) GR subcellular location was detected in the SIRT2 overexpression or SIRT2 knocked‐down cells, with β‐actin and lamin B served as markers for the fractions of cytoplasm and nucleus, respectively. SIRT2 overexpression increased the relative expression of GR in the nucleus compared with SIRT2 knock‐down. Nuclei isolation was repeated at least three times, and a representative immunoblot is shown. (D) To examine the effect of SIRT2 on the binding capacity of GR to glucocorticoid receptor response element (GRE), GR‐driven dual‐luciferase reporter assay was performed in the B104 cells, in which rSIRT2 was overexpressed or knocked‐down, 50 as described in the Materials and Methods. When rSIRT2 was overexpressed, expression of the reporter gene was significantly up‐regulated compared with rSIRT2 was knocked‐down or control cells (each experiment was performed in triplicate and represented as mean ± SEM; t test, *P < 0.05 or **P < 0.01). (E) The B104 cell or with SIRT2 overexpression or with SIRT2 knocked‐down were treated with 10 μmol/L of Dx for 12 h. The cell lysate was incubated with anti‐Hsp90 antibody to immunoprecipitate total Hsp90 protein. Subsequently, the acetylated Hsp90 was detected in the complex through Western blot
