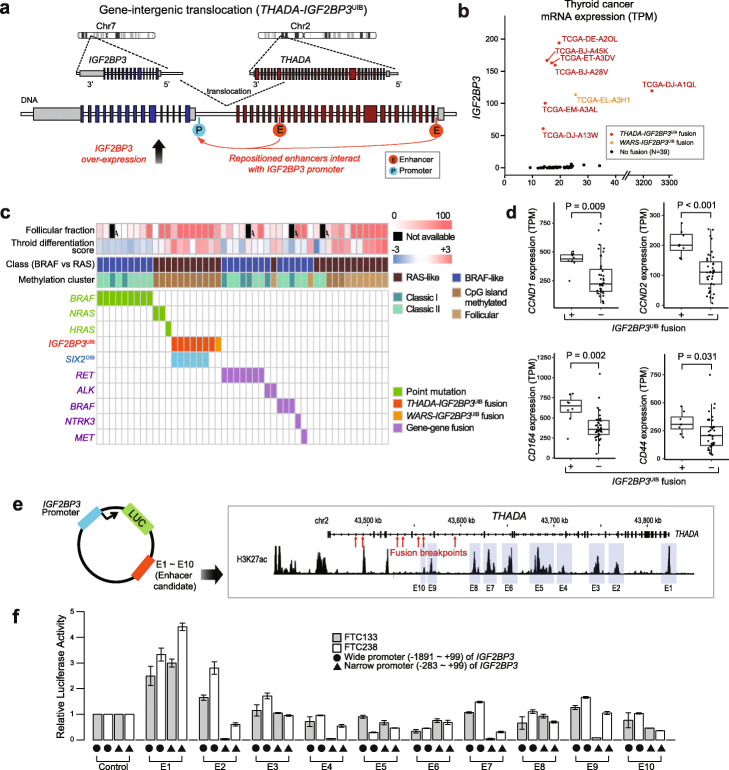
Fig. 4.
Computational and experimental characterization of THADA-IGF2BP3UIB. a This THADA-IGF2BP3UIB fusion was produced as a result of a translocation between chromosomes 2 and 7. RNA-seq data show the upregulation of intact IGF2BP3 mRNAs, suggesting the repositioning of a regulatory element for IGF2BP3. b The expression of IGF2BP3 is dramatically increased for the eight fusion cases (seven THADA-IGF2BP3UIB and one WARS-IGF2BP3UIB). TPM, transcripts per million. c The presence of IGF2BP3UIB fusion (red) was mutually exclusive with other tumor-driving mutations for all eight cases. The IGF2BP3UIB fusion group showed similar molecular and pathologic signatures to HRAS or NRAS mutation cases, as reflected in high follicular fraction, RAS-like class, CpG island-methylated pattern, and positive thyroid differentiation score. Note that six cases with IGF2BP3UIB and SIX2DIB fusions were confirmed by whole-genome sequencing. dCCND1, CCND2, CD164, and CD44, which are known to be regulated by IGF2BP3 in various cancers, are differentially expressed between the 8 IGF2BP3UIB fusion-positive and 47 fusion-negative thyroid samples. e A vector design (left) to investigate the interaction between the IGF2BP3 promoter and potential enhancer regions. Ten regions in THADA with H3K27ac peaks were tested (right). f Dual-luciferase reporter assays using a wide and a narrow IGF2BP3 promoter in two thyroid cancer cell lines (FTC133 and FTC238) show that two regions (E1 and E2) could interact with the wide promoter and one region (E2) could interact with the narrow promoter. Error bars indicate standard deviations