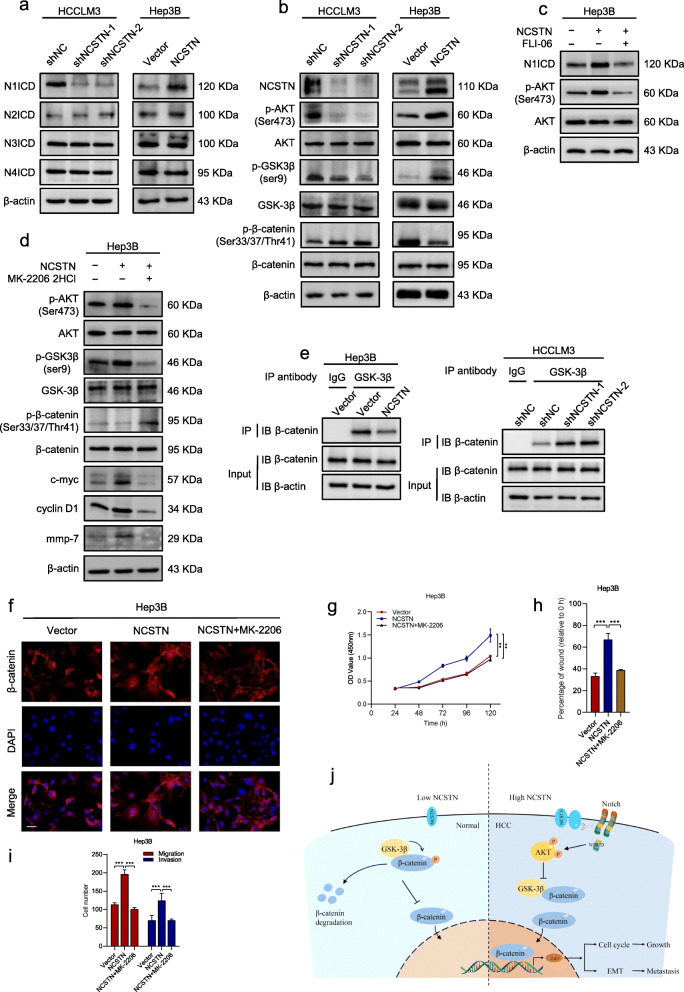
Fig. 6.
NCSTN regulates β-catenin through Notch/AKT/GSK-3β signaling pathway. a The expression of N1ICD, N2ICD, N3ICD, and N4ICD in the indicated HCCLM3 and Hep3B cells. b Representative immunoblot analyses of total AKT, phosphorylated AKT (Ser473), GSK-3β, phosphorylated GSK-3β (ser9), β-catenin and phosphorylated β-catenin (Ser33/37/Thr41) in the indicated HCC cells. c The expression of N1ICD, AKT and p-AKT in the indicated Hep3B cells, treated with FLI-06 (the Notch inhibitor, 5 μM, 24 h). d Representative immunoblot analyses of AKT, p-AKT (Ser473), GSK-3β, p-GSK-3β (ser9), β-catenin and p-β-catenin (Ser33/37/Thr41) in the indicated Hep3B cells, treated with MK-2206 2HCl (the AKT inhibitor, 10 μM, 24 h). e Co-IP assays showed NCSTN regulated the interaction between GSK-3β and β-catenin in the indicated cells. f Immunofluorescence assays showed NCSTN-mediated nuclear translocation of β-catenin was rescued in cells treated with MK-2206 2HCl. g CCK8 assays showed that NCSTN-mediated cell growth could be rescued in Hep3B cells treated with MK-2206 2HCl. h, i The migration and invasion capacity was rescued in cells treated with MK-2206 2HCl. j The propose model showing the regulatory landscape of NCSTN/Notch/AKT/β-catenin signaling axis in promoting cell growth and metastasis of HCC. Loading control was evaluated by β-actin. *p < 0.05, **p < 0.01, ***p < 0.001