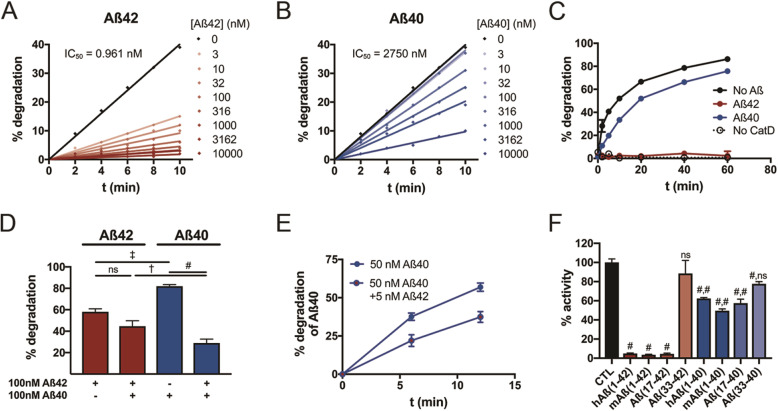
Fig. 4.
Aβ42 is a potent competitive inhibitor of CatD. a, b Competitive inhibition by Aβ42 (a) and Aβ40 (b) of CatD activity quantified by a fluorogenic substrate. Note that just 3 nM Aβ42 inhibits CatD (nominal concentration, ~ 1 nM) by more than 50%. c Comparable data for CatD activity quantified using an Aβ degradation assay, with 200 nM fluorescent Aβ alone (no Aβ) or in combination with 200 nM Aβ42 (red) or Aβ40 (blue). d Quantification of Aβ42 (red) and Aβ40 (blue) degradation either alone (100 nM) or in combination (100 nM each). Note that Aβ42 significantly inhibits Aβ40 degradation, but the converse is not true. Data are mean ± SEM for 4–8 replicates per group. †p < 0.01; ‡p < 0.001; #p < 0.0001. e Aβ40 degradation is significantly inhibited by 1/10 the concentration of Aβ42, a ratio representative of that present in vivo. f CatD is strongly inhibited by multiple Aβ peptides and fragments ending at position 42, including full-length murine Aβ (mAβ(1–42)) and the p3 fragment (Aβ(17–42)), more strongly than the corresponding peptides ending in Aβ40. The C-terminal fragment of Aβ42, Aβ(33–42), failed to inhibit significantly, while the corresponding fragment, Aβ(33–40), showed a modest but statistically significant inhibition under the conditions tested. Data are mean ± SEM for 4–8 replicates per group. #p < 0.0001. For data on Aβ peptides ending at position 40, the 2 symbols reflect the statistical significance of comparisons to buffer-only control (CTL) and to the corresponding fragments ending at position 42, respectively