Fig. 1: Structure of the heterotetrameric human ESCRT-I head.
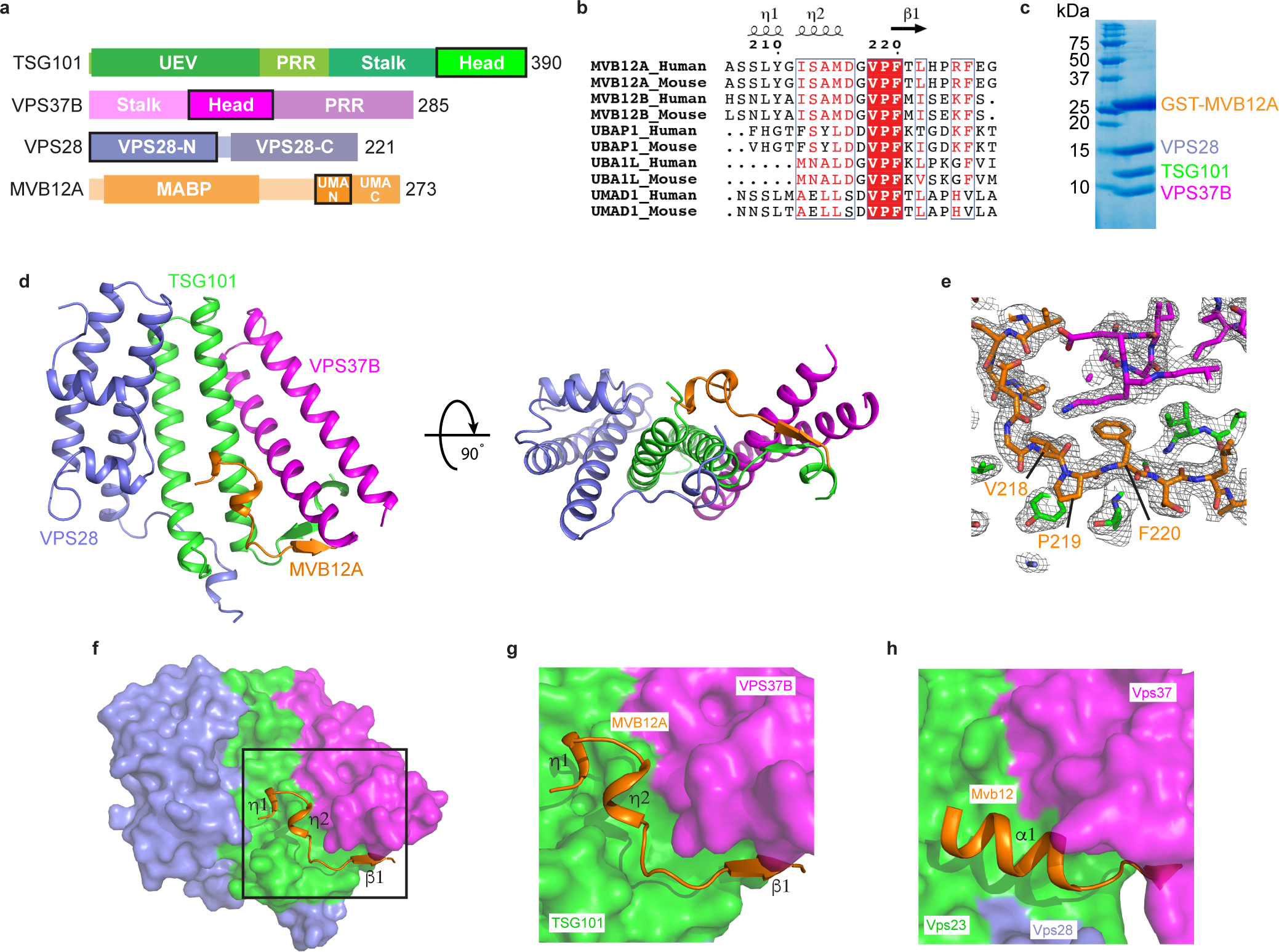
a, Domain architecture of human ESCRT-I subunits. Constructs used for structural analysis are highlighted. b, Sequence alignment of MVB12A paralogs and orthologs. Secondary structure displayed above the alignment is derived from the human ESCRT-I head structure. Alignment was generated using ClustalW and ESPript. c, GST-tagged MVB12A 206–228 was co-expressed with trimeric ESCRT-I head (TSG101 308–388, VPS37B 97–167, VPS28 1–122) in E.coli and analyzed by SDS-PAGE. Lysate was incubated with glutathione beads and complex integrity analyzed by SDS-PAGE following multiple washes. d, Ribbon diagram of the human ESCRT-I head crystal structure. e, MVB12A 2Fo-Fc omit map. MVB12A chain was removed and model re-refined. Resulting 2Fo-Fc map is contoured at 1.0 σ and represented as a grey mesh. MVB12A chain replaced for clarity. f, Surface representation of the human ESCRT-I head with MVB12A shown as an orange ribbon. g, Enlargement of boxed region shown in (f). h, Mvb12 binding site from the previously determined heterotetrameric yeast ESCRT-I head (PDB 2P22). Mvb12, Vps37, Vps23 and Vps28 are colored orange, magenta, green and purple respectively. Uncropped image for panel (c) is in Supplementary Figure 1.
