Fig. 3: Helical ESCRT-I head assemblies.
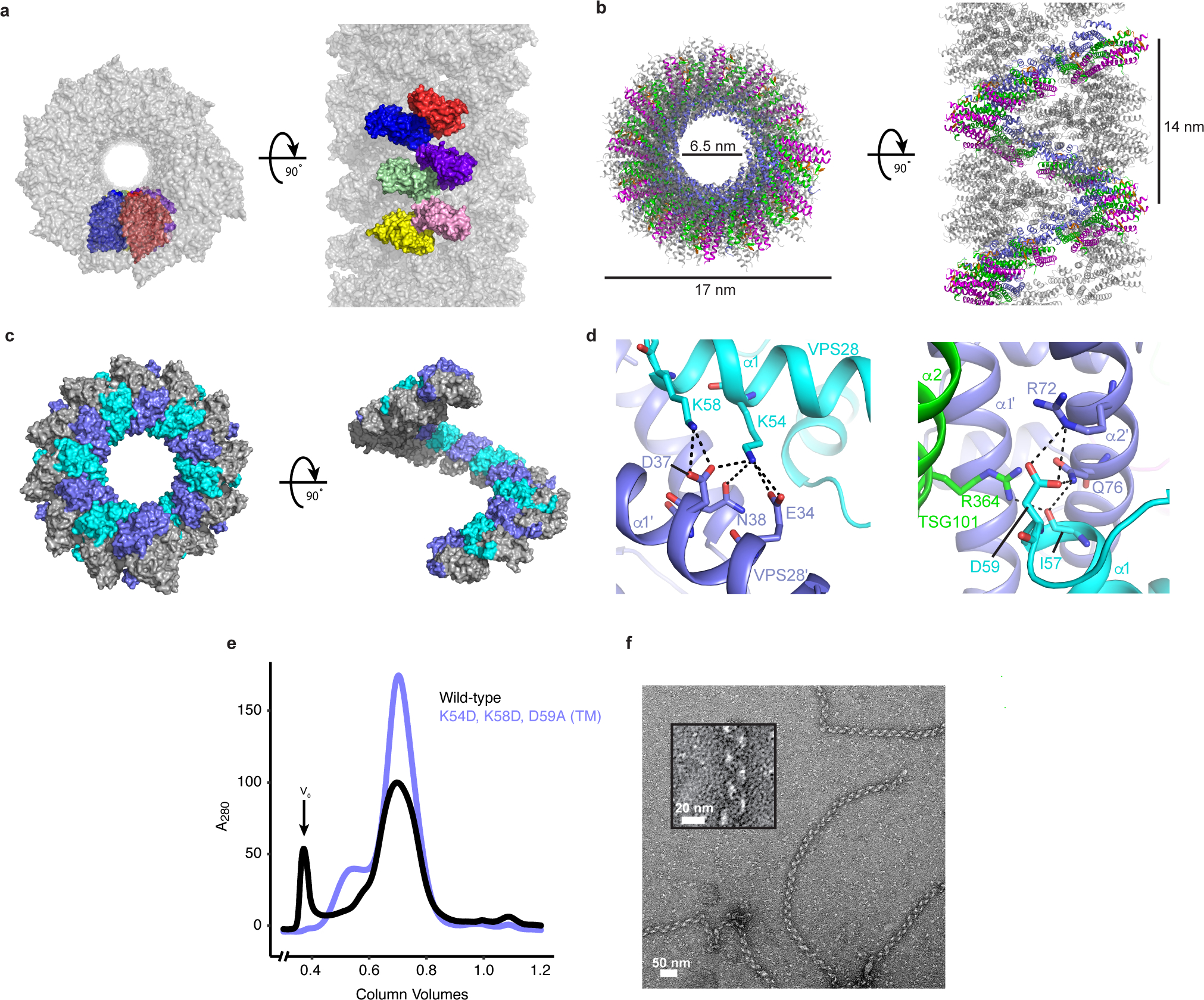
a, The crystal from which the ESCRT-I head structure was determined consists of a series of helical tubes, one of which has been isolated and shown as a surface representation. The crystal exhibits P61 symmetry with 6 copies of the tetrameric ESCRT-I head in the asymmetric unit. An individual asymmetric unit is highlighted and each ESCRT-I head protomer assigned a distinct color for clarity. b, Tube composed of three interleaved ESCRT-I head helices. One continuous helix is colored for clarity. TSG101, VPS28, VPS37B, MVB12A are colored green, purple, magenta and orange respectively. Tube dimensions are labelled. c, Surface representation of a single, continuous ESCRT-I helix. The intra-helical interface is primarily composed of residues from VPS28. Individual VPS28 subunits are colored either purple or cyan in order to clearly distinguish surface boundaries. d, Detailed views of the helical interface highlighting key residues. Inter-protomer bonds depicted with dashed lines. Subunits are colored as in (c). e, Size-exclusion chromatograms of WT and mutant (VPS28 K54D, K58D, D59A) binary (TSG101–VPS28) ESCRT-I head subcomplexes. Proteins were expressed in E.coli and subjected to initial IMAC purification prior to being applied on a size-exclusion column. Void volume (V0) labelled accordingly. Chromatograms are representative of 3 independent biological replicates. f, Electron micrograph of WT ESCRT-I filaments obtained from the pooled V0 fraction shown in (e). Filaments were observed for wild-type but were not observed for the mutant construct in three biological replicates. Zoomed view of a typical WT ESCRT-I filament shown inset.
