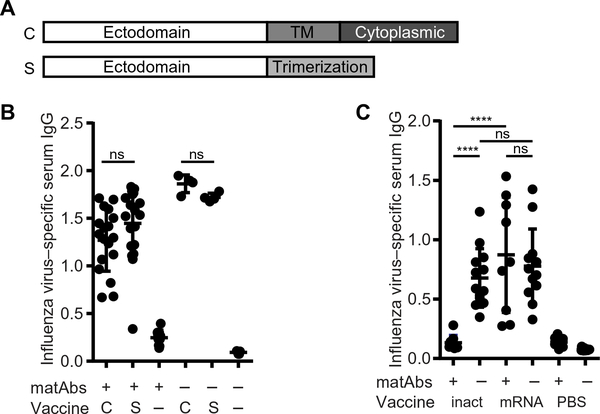
Fig. 4. Cell-associated and secreted PR8 HA mRNA-LNP vaccines elicit similar antibody responses, and a low dose of mRNA-LNP vaccine overcomes matAb inhibition.
(A) The schematic shows PR8 influenza virus hemagglutinin (HA) mRNA constructs. mRNA expressing full-length PR8 HA produced cell-associated (C) HA. For some experiments, we used mRNA expressing secreted (S) HA. For this construct, the transmembrane (TM) and cytoplasmic domains were removed, and a trimerization domain was introduced. (B) Mice (21 days old) were vaccinated with cell-associated (C) or secreted (S) PR8 influenza virus HA mRNA-LNP vaccine or PBS (−) in the presence or absence of influenza virus–specific matAbs. Serum was collected 70 days post-vaccination, and influenza virus–specific IgG was measured by ELISA. n = 4 (−matAbs/C vaccine and −matAbs/S vaccine), n = 5 (naïve mice), n = 10 (+matAbs/PBS), n = 18 (+matAbs/C vaccine), or n = 19 (+matAbs/S vaccine) mice per group. (C) Mice (21 days old) were intramuscularly vaccinated with 1000 hemagglutination units of purified inactivated PR8 influenza virus (inact), 0.3 μg of PR8 influenza virus HA mRNA-LNP vaccine (mRNA), or PBS as control in the presence or absence of PR8 influenza virus–specific matAbs. Serum was collected 70 days post-vaccination, and influenza virus–specific serum IgG was measured by ELISA. Data are pooled from two independent experiments (n = 9 to 15 mice per group). Each point represents one mouse. Data are shown as means ± SD. Titers were compared by one-way ANOVA with Sidak’s (B) or Tukey’s (C) post hoc test. ****P < 0.0001. ns, not significant. Panel (B) shows results of two independent experiments. ns, not significant.