Fig. 1 |. Conformational landscape of α-synuclein.
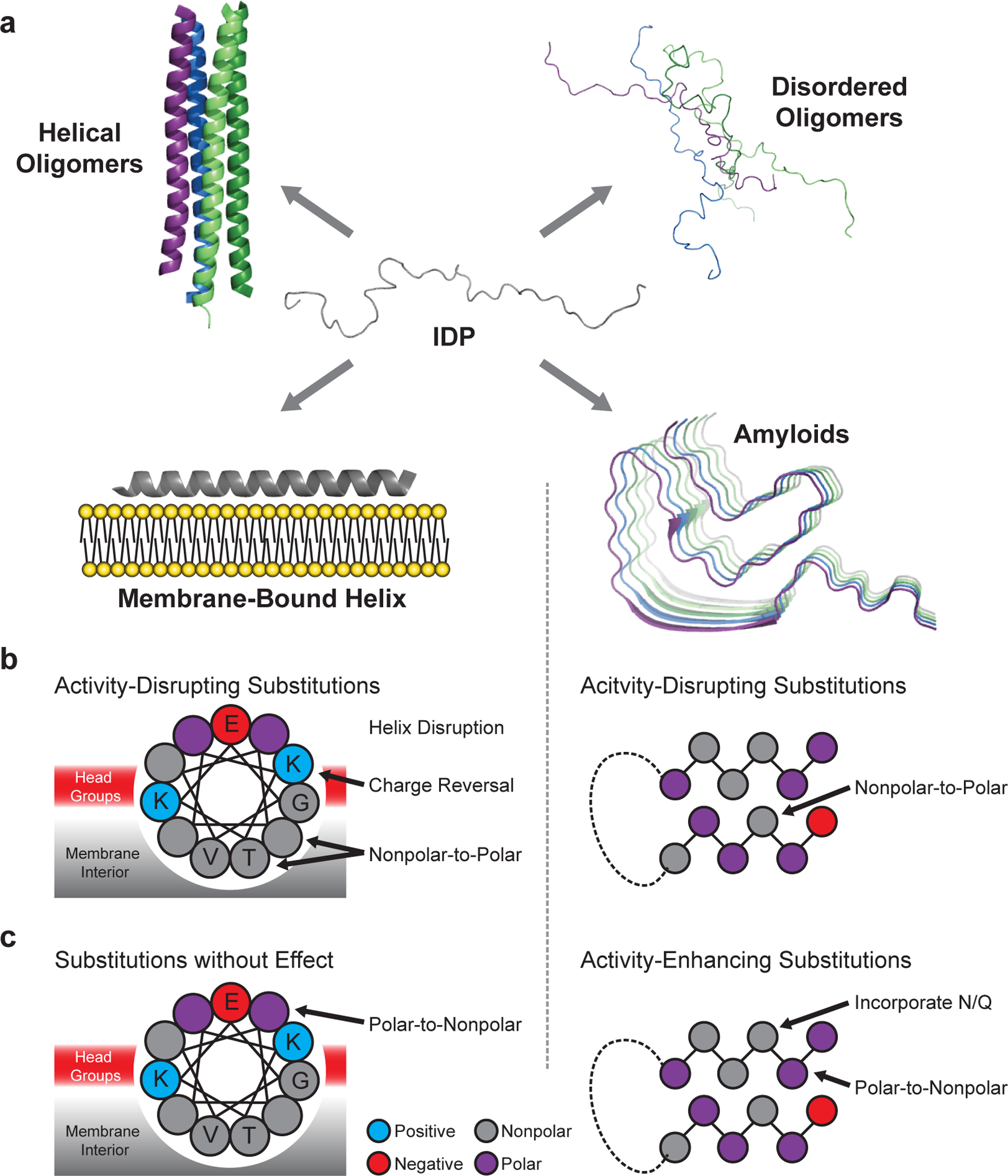
a, α-Synuclein adopts intrinsically disordered conformations10,11, poorly structured and/or helix-rich oligomers12,13, a variety of membrane-bound helical states14–16, and amyloid-like conformations with distinct cross-β structures and disordered regions17–19. Amino acid substitutions are predicted to differentially perturb each structure, allowing for model discrimination by mutational scanning. b, Substitutions predicted to disrupt the activity of the membrane-bound helix (left) and the amyloid (right) structures. c, Substitutions predicted to enhance or have little effect on activity. Mutations affecting other possible active states are not shown, but discussed below.
