Fig. 3 |. Mutational sensitivity varies by depth of membrane burial.
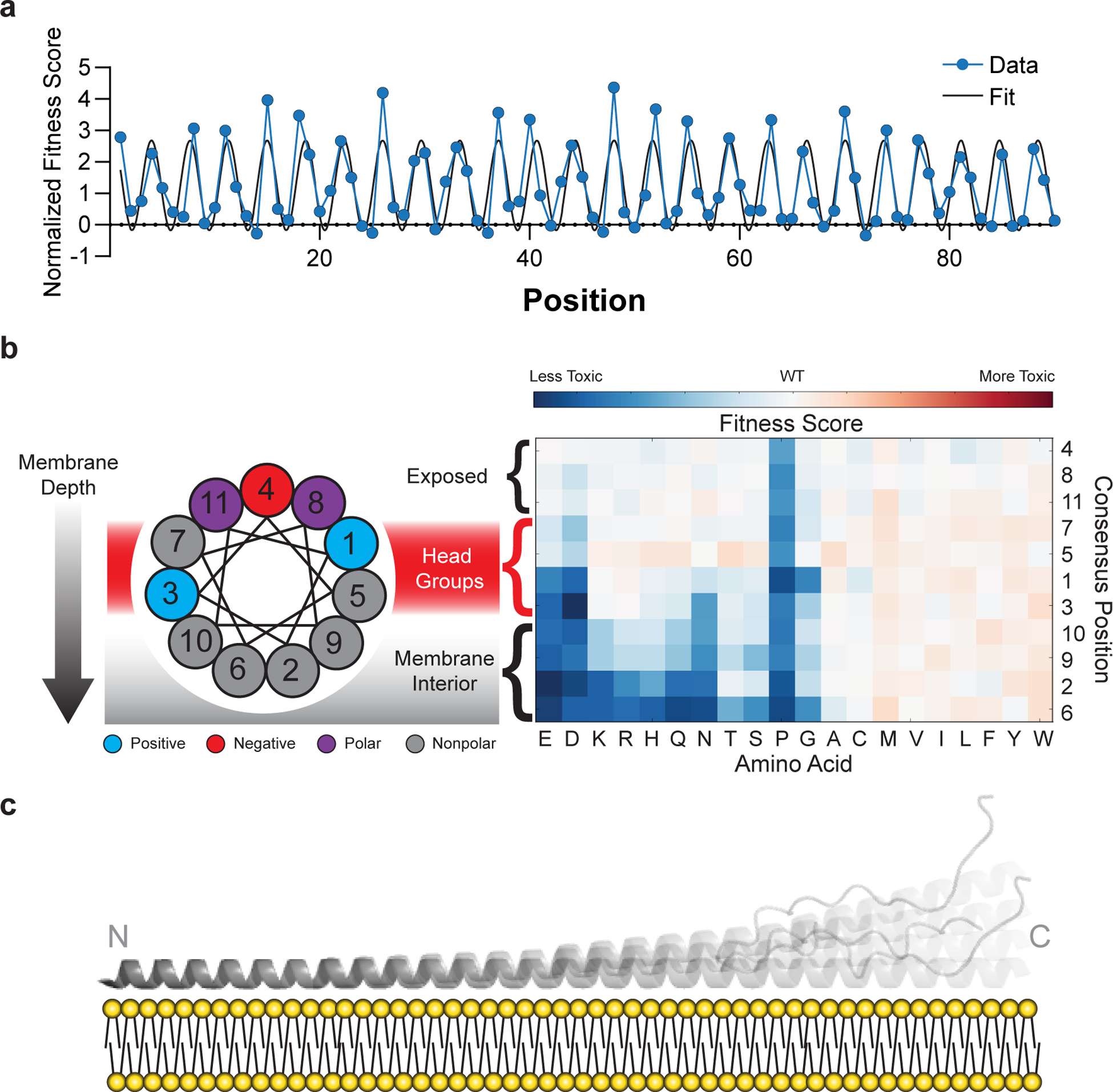
a, Average fitness scores of mutations to polar (S, T, N, Q, H, R, K, D, E) residues as a function of position, normalized against an 11-residue window average (Fig. 2C). A sinusoid fit describes the data as having a periodicity of 3.67 ± 0.01 residues (95% CI). b, Fitness scores were averaged over equivalent positions in each of the seven 11-residue repeating segments and ordered by predicted depth of membrane penetration. c, Structural model of α-synuclein as a single amphiphilic helix interacting with a lipid bilayer, demonstrating increased dynamics toward the C terminus, which is consistent with data from deep mutational scanning, EPR spectroscopy15, and NMR spectroscopy16,33.
