Figure 3.
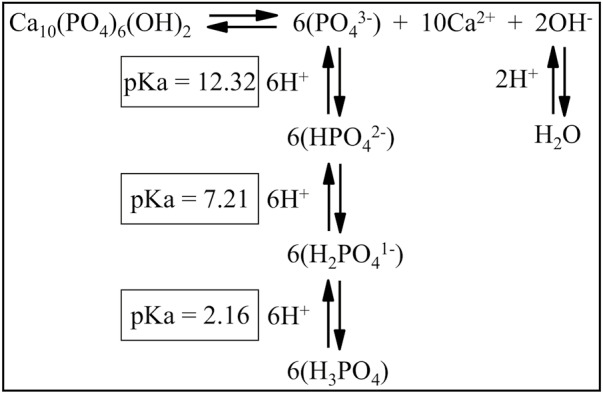
Interaction of hydroxyapatite with ions in solution. Hydroxyapatite (HAP) demineralization and remineralization reactions are reversible and depend on the concentrations of ions in the solution surrounding the crystal. The negatively charged ions in solution that maintain the ion product (Qsp) above or equal to the solubility product constant (Ksp) of hydroxyapatite are reduced in concentration by a drop in pH. When the pH of the solution in contact with HAP falls from 7 to 5, the [OH–] falls from 10–7 to 10–9 M (is reduced by 10–2), reducing the Qsp by (10–2)2 or 10–4. The [PO43–] falls even further, from an original concentration of N, to 1.6 × 10–4 × N, reducing the Qsp by (1.6 × 10–4)6 or 1.7 × 10–23. The total fall in Qsp from the [OH–] and [PO43–] would be 1.7 × 10–27. If this reduced the Qsp below the Ksp for HAP (5.5 × 10–118), then HAP would start to demineralize. A similar drop in pH for fluorapatite barely affects [F–], so the fall in the Qsp of fluorapatite would be that caused by the drop in [PO43–], or by 1.7 × 10–23. If this reduced the Qsp below the lower Ksp for FAP (5.0 × 10–123), then the FAP would start to demineralize
