Abstract
Background:
People living in coastal communities are at risk for exposure to environmental hazards, including legacy chemicals. We can use databases such as NHANES to assess whether contaminants in coastal communities are present in higher levels than in the United States overall. We can use information from studies of local animal populations to assess which of these contaminants could have been transferred to people from their shared environment.
Objective:
Our objectives were to examine the POP profiles in human populations in areas where there are published POP profiles in resident dolphins and to compare our results with data from NHANES and the dolphin studies.
Methods:
We identified three areas where POPs have been analyzed in local resident dolphin populations (total N =73). We identified human communities in the same areas, and asked 27 eligible adults to read and sign a consent form, complete a questionnaire about demographics and seafood consumption, provide nine 10-mL blood samples, and provide one sample of seafood (N = 33). Blood and seafood were analyzed for a suite of POPs similar to those analyzed in published dolphin population studies. We compared the results from human blood analyses with NHANES and with data from the published reports of dolphin studies.
Results:
Levels and proportions of specific POPs found in people and animals reflect POPs found in the local environment. Compared with the nationally representative data reported in NHANES, the levels of many POPs found in high levels in dolphins were also higher in the corresponding human communities.
Conclusions:
Contaminants measured in marine animals, such as dolphins, can be used to identify the types and relative levels of environmental contaminants expected to occur in people sharing the same environment. Likewise, contaminants measured in coastal human populations can provide insight into which contaminants may be found in nearby animal populations.
Keywords: Ocean toxins, Sentinel animals, Marine mammals, Biomonitoring
GRAPHICAL ABSTRACT
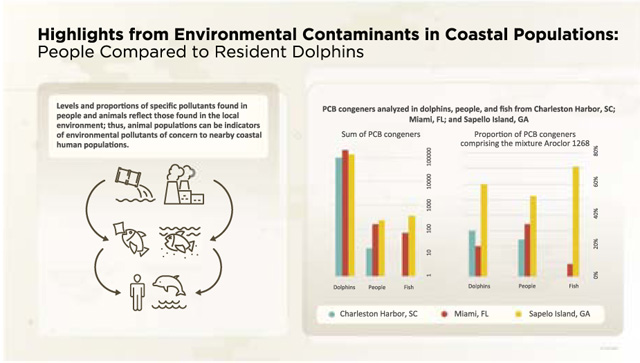
1. Introduction
Coastal communities are at risk for a number of environmental hazards, including exposure to legacy chemicals found in local fauna, flora, sediment, air, and water. Many studies have linked consumption of marine-based food sources with human exposure to environmental contaminants in maritime communities, including communities on the Faroe Islands and Seychelles Islands and in the Inuit (see review by Dewailly et al., 2008). Tang-Péronard et al. (2014) suggested that children from the Faroe Islands were at increased risk for obesity from prenatal exposure to PCBs1 and DDE. The Seychelles child development study evaluated the effects of in utero exposure to methyl mercury (Myers et al., 2003). Although they did not identify an associated neurodevelopment risk, the investigators documented prenatal exposure to methyl mercury. Boucher et al. (2014) reported adverse effects on cognitive development of Inuit infants from prenatal exposure to PCBs, methyl mercury, and lead. Exposure from environmental contaminants accumulating in seafood is not limited to maritime communities, however. Recreational fishers and Tribal communities who consume fish from the Great Lakes are also exposed to environmental contaminants and may be at increased risk for diabetes (Turyk et al., 2009) and other diseases (Turyk et al., 2012). More recently, Fair et al. (2018) examined environmental contaminants in fish from Charleston Harbor, South Carolina, and found levels of some POPs exceeding current EPA human screening values for human cancer risks.
The association between environmental contaminants in food sources and subsequent exposures is not limited to humans. The National Oceanic and Atmospheric Administration (NOAA) conducted studies on resident common bottlenose dolphins (Tursiops truncatus), hereafter referred to as dolphins, in Biscayne Bay, Miami, Florida; Sapelo, Georgia; and Charleston, South Carolina. The studies confirmed that dolphins are exposed to a location-specific suite of contaminants through the food web and that some of these contaminants were present in higher levels compared with dolphins in other regions in the Southeast United States. Litz et al. (2007) analyzed dart biopsy blubber samples from Biscayne Bay, Florida dolphins and found differences in persistent organic pollutant (POP) concentrations related to geographic distribution of the dolphins. For example, total PCBs were five times higher in males with sighting histories in northern areas than in those with sighting histories in southern areas of the bay, demonstrating that habitats influence the specific contaminant concentrations in these mammals. Kucklick et al. (2011) compiled data from dolphin specimens from 14 sites in the U.S. Atlantic and the Gulf of Mexico including rural and urban estuaries near a Superfund site in Brunswick, Georgia. POP profiles varied by location, and the profiles from dolphins sampled near the Brunswick and Sapelo, Georgia estuaries were significantly different from others in the study. For example, PCB congeners from Aroclor 1268 were the most predominant in dolphins sampled in the estuaries near an area with historic Aroclor 1268 contamination, reflecting widespread contamination by this specific PCB mixture in these estuaries as noted by Maruya (1997). Finally, Fair et al. (2012) found high levels of perfluorinated alkyl substances (PFAS) in plasma of dolphins from the Indian River Lagoon, Florida, and Charleston Harbor, South Carolina. The differences in PFAS congener mixtures and concentrations suggested that the pollutants accumulating in these animals reflect site-specific contamination.
Animals, including wild animals, livestock, and pets, can serve as sentinels for human exposure to and health effects from environmental contaminants (e.g., Rabinowitz et al., 2009; Bossart, 2010; Reif, 2011; Buttke, 2011). Because humans and other animals can be exposed to environmental contaminants, for example, through their food, we can use test results from local animal populations to reveal clues about what to expect in people sharing the same environment.
One example of applying animal test results to a relevant human population was a study by Reif et al. (2015) in which they found elevated blood mercury levels in the dolphins of the Indian River Lagoon in Florida and hypothesized that the coastal human population might also be exposed to mercury. When they examined hair samples from local residents, they found elevated hair mercury levels that were associated with both the frequency of eating seafood and the proportion of seafood obtained from the Lagoon. As noted by Bossart (2010), dolphin populations could likely serve as sentinels for other coastal environmental public health hazards, including long-term exposures to POPs.
We examined POPs in three coastal communities where subsistence fishers and others consume local seafood and where there were data on environmental contaminants in the blood or blubber of nearby dolphin populations. Our objectives were to examine the environmental contaminant profiles in coastal human populations in areas where these contaminants have been found in resident dolphins and compare our results with data from NHANES and the dolphin studies.
2. Methods
2.1. Participant recruitment for three studies
Our study was approved by the Institutional Review Boards of the Centers for Disease Control and Prevention (CDC) and the Medical University of South Carolina (for Charleston Harbor). We used convenience samples.
2.1.1. Sapelo Island, Georgia
We collaborated with the Research Coordinator for the Sapelo Island National Estuarine Research Reserve (SINERR), who already had a trusted relationship with the community. We recruited nine individuals who were at least 18 years of age, who responded to posted flyers about the study, had lived on Sapelo Island for at least five years, and who ate at least two meals each week over the last two years that included seafood from the waters near Sapelo Island.
2.1.2. Miami, Florida
We collaborated with investigators from the National Oceanic and Atmospheric Administration (NOAA) to recruit nine individuals who were at least 18 years of age, were fishing from the Rickenbacker Causeway bridge pier during our recruiting days, and who ate at least two meals each week over the last two years that included seafood from the Miami area.
2.1.3. Charleston Harbor, South Carolina
We collaborated with physicians at the Medical University of South Carolina (MUSC) to recruit nine women who presented for prenatal care at the MUSC hospital during our recruiting period, were at least 18 years of age, who received prenatal care at the Medical University of South Carolina, were in their first trimester of their first pregnancy, and who ate at least two meals each week over the last two years that included seafood from Charleston Harbor and surrounding areas.
2.2. Study activities
We asked a total of 27 eligible adults to do the following activities: read and sign a consent form to participate in the study and complete a study questionnaire in October 2010 (Sapelo Island and Miami) or November 2010 (Charleston Harbor). The questionnaire asked questions about demographics, hunting and fishing practices, and seafood consumption. Each study participant provided a sample of freshly-caught local seafood.
A certified phlebotomist collected nine 10-mL venous blood specimens from each participant. Blood specimens were collected, processed, and handled as specified in the NHANES MEC Laboratory Procedures Manual (CDC, 2016).
2.3. Seafood sample handling
Locally caught seafood provided by the study participants for these studies were cataloged using a unique number per sample. In Miami, in addition to the fish provided by study participants, NOAA staff collected seven fish (6 pinfish, 1 mutton snapper) from the fishing pier on the same day as our study. We analyzed 8 fish fillets from Sapelo Island, 17 from Miami, and 8 samples of seafood (crab and shrimp) from Charleston Harbor.
Samples were handled with nitrile gloves, placed on pesticide residue analysis-grade hexane-rinsed aluminum foil, and lengths and weights recorded when appropriate. All samples were then wrapped in pesticide-grade hexane-rinsed aluminum foil, placed in plastic bags, and maintained at −20 °C for the duration of the field sampling and then stored at −80 °C. Samples were placed on dry ice during transport to the National Institute of Standards and Technology (NIST; Charleston, SC). Upon arrival, samples were held at −80 °C until analyzed.
2.4. Laboratory methods
Human blood samples were analyzed by the CDC Division of Laboratory Sciences (DLS) and fish samples were analyzed by Pace Analytical Laboratory and NIST with methods summarized here. CDC’s DLS used methods described in the National Report on Human Exposure to Environmental Chemicals, Updated Tables, March 2018 (CDC, 2018) to analyze contaminants in human specimens. Specific methods include CDC (2003) (trace elements); Jones et al., 2012 (preparation of serum samples), Kuklenyik et al., 2005; Kato et al., 2011a (PFAS); Patterson Jr. et al., 1991 (dioxins, furans), and Sjödin et al., 2004 (PCBs, PBDEs).
NIST used the method described in Litz et al. (2007) to analyze organochlorine pesticides, PCBs, and PBDEs in fish samples with some modifications. Fish were removed from the −80 °C freezer, placed on a clean Teflon™ cutting board, and allowed to thaw for approximately 5 min. Fish were filleted, and then ground in a stainless steel meat grinder (Waring Pro, MG800, Lancaster, PA) by passing the sample at least twice through the grinder. The homogenate was collected in a stainless steel container, mixed manually with a hexane-rinsed spatula and then transferred into a glass jar with Teflon™ lined lid and stored in a −80 °C freezer. Prior to analysis, the glass jar was thawed to room temperature then 4 g of tissue was analyzed as outlined in Litz et al. (2007). Standard Reference Material (SRM) 1947 Lake Michigan Fish Tissue was analyzed along with fish samples as a control. The material has certified values for a variety of organohalogen contaminants. Pace Analytical Laboratory used EPA Methods 6020 (EPA, 2014) to analyze trace elements in fish samples and EPA method 3545 (EPA, 1997) to extract POPS, EPA method 8290A (EPA, 2007) to analyze dioxins, and EPA method 8082a (EPA, 2007a) to analyze PCBs in fish samples provided to them by NIST.
2.5. Dolphin data
With exception of PFAS levels from dolphins collected near Sapelo Island (Jessica Reiner, National Institute of Standards and Technology, unpublished), all dolphin data are published elsewhere: Sapelo Island (Balmer et al., 2011, n = 27), Charleston Harbor (Kucklick et al., 2011, and Fair et al., 2012, n = 20), and Biscayne Bay, Miami (Litz et al., 2007, n = 16). The results represent analysis of plasma samples for PFAS and blubber samples for the other analytes.
2.6. Statistical analyses
We used SAS version 9.4 (SAS Institute Inc., Cary, NC) to calculate univariate statistics. Results are presented for contaminants that were measured above the analytic limit of detection LOD (see Supplemental Table for LOD values) in at least 60% of the human specimens or fish samples. We reported medians and ranges, thus there were no substitutions for values <LOD, which were in the dataset as zeros.
2.6.1. Comparisons
We compared the data from human specimens with the 50th and 95th percentiles from the National Health and Nutrition Examination Survey (NHANES) as follows: NHANES 2003–2004 for the persistent compounds, because after 2004, measurements of these chemicals were made in pools of serum and are not comparable to individual sample results; NHANES 2009–2010 for metals because samples were analyzed for metals in 2010; and NHANES 2011–2012 for selenium because this was the first cycle in which this metal was measured (CDC, 2018). In an attempt to match the demographics of our three study populations with NHANES populations, we used NHANES data for all males for the Sapelo Island participants, data for all NHANES participants (data include males and females) for the Miami participants, and data for all females for the Charleston Harbor participants. See Supplemental Table 2 for NHANES 50th and 95th percentiles and 95% confidence intervals for these population categories within the noted specific survey years.
We compared data from our study participants with data from the seafood samples they provided and with published data from the resident dolphins of Sapelo Island (Balmer et al., 2011, n = 27), Charleston Harbor (Kucklick et al., 2011, and Fair et al., 2012, n = 20), and Biscayne Bay, Miami (Litz et al., 2007, n = 16).
3. Results
3.1. Study participant demographics and fish consumption
Study participants’ demographics and fish consumption information are in Table 1. All study participants confirmed eating seafood they caught themselves at least two to three times per week for at least the last two years. Participants from Sapelo Island and Biscayne Bay reported eating seafood that was likely locally caught. For Charleston, although participants provided locally-caught seafood (shrimp) for our study, most of the seafood these participants reported eating (e.g., catfish, bass, sardines, tilapia, and mahi mahi) was not associated with the Charleston Harbor area and was likely purchased from fish markets. Of the nine participants in Miami, seven indicated they were unemployed and were likely relying on local fish as a primary food source.
Table 1.
Selected demographic characteristics and types of fish study participants reported eating.
| Sapelo Island | Miami | Charleston | |
|---|---|---|---|
| Number of study participants | 9 | 9 | 9 |
| Number (percent) female | 1(11%) | 1(11%) | 9(100%) |
| Age in years: median (range) | 51(21–74) | 52(22–57) | 24 (18–35) |
| Current smokers | 4(44%) | 8(90%) | 0(0%) |
| Race/ethnicity (number, percent)White | 3(33%) | 0 | 4(44%) |
| Black | 6(67%) | 0 | 5(56%) |
| Hispanic | 0 | 9(100%) | 0 |
| Self-reported fish eatena | Brim/spot | Bluefish | Bass |
| Catfish | Chopa (chub) | Bream | |
| Clams | Clams | Catfish | |
| Crawfish | Cojinua (jack) | Clams | |
| Croaker | Crabs | Crabs | |
| Flounder | Crawfish (spiny lobster) | Flounder | |
| Mullet | Croaker | Grouper | |
| Oysters | Cubera (snapper) | Mahi mahi | |
| Red drum | Jiguagua (jack) | Oysters | |
| Shark | Ladyfish | Sardines | |
| Sheepshead | Lorro (parrotfish) | Shrimp | |
| Shrimp | Mullet | Snapper | |
| Silver perch | Oysters | Tilapia | |
| Southern kingfish | Pargo (snapper) | Tile fish | |
| Spottail bass/red fish | Picuda (barracuda) | Tuna | |
| Spotted sea trout Whiting | Ronco (grunt) Sardines Shrimp Snapper Snook Tiberun (shark) Tracha (mackerel) |
Whiting |
Translations from Spanish from: Speardiver, Mexfish.com.
3.2. Contaminants
The contaminant concentrations in human blood specimens, dolphin blood or blubber specimens, and fish samples are in Tables 2, 3, and 4, respectively. The seafood samples from participants in Charleston Harbor were crabs or shrimp. And did not have detectable levels of any of the contaminants we tested for; thus, we present no data for these samples in Table 4.
Table 2.
Whole blood (trace elements) or serum concentrations (PBDEs, chlorinated pesticides, dioxins, PFASs, and PCBs) in study participants. Medians from human blood or serum specimens are reported only if the analyte was detected in blood or serum from at least five of the nine participants. Comparison values from NHANES (CDC, 2018) are in Supplemental Table 2; comparison values are from all NHANES men for Sapelo Island, all NHANES (men and women) for Miami, and all NHANES women for Charleston Harbor. Italics indicate the value is >50th percentile from NHANES and bold indicates the value is >95th percentile from NHANES. Values are ng/g lipid unless otherwise specified.
| Analyte | Sapelo Island (N = 9) Median (range) | Miami (N = 9) Median (range) | Charleston (N = 9) Median (range) |
|---|---|---|---|
| Metals μg/L | |||
| Cadmium (μg/L) | 0.39 (023–1.6) | 0.87 (0.36–1.69) | 0.2 (<LOD-0.4)b |
| Lead (μg/dL) | 3 (1.7–7.7) | 8.96 (2.35–25.0) | 0.6 (0.3–1.0) |
| Mercury (μg/L) | 3.2 (2.4–9.4) | 6.46 (2.22–40.1) | 1.1 (0.5–4.1) |
| Selenium (μg/L) | 119 (106–137) | 111 (77.0–159) | 124(107.6–173.3) |
| PBDEs | |||
| PBDE17 | a | a | a |
| PBDE 28 | a | 0.60 (<LOD-7.20) | 0.7 (<LOD-2.4) |
| PBDE 47 | 11.8 (3.0–65.3) | 8.50 (2.40–98.4) | 10.5 (1.2–30.8) |
| PBDE 66 | a | a | a |
| PBDE 85 | a | a | a |
| PBDE 99 | 2.0 (0.7–32.9) | 3.10 (0.60–25.7) | 2.0 (<L0D-9.5) |
| PBDE 100 | 2.9 (1.0–24.1) | 2.10 (0.60–18.8) | 2.4 (0.3–5.0) |
| PBDE 153 | 7.2 (1.36–11.9) | 11.7 (2.20–33.2) | 4.4 (1.4–22.3) |
| PBDE 154 | a | a | a |
| PBDE 183 | a | 0.60 (<L0D-1.50) | a |
| Median sum of congeners 47,99,100,153, and 154 | 24.6 | 26.7 | 16.6 |
| Chlorinated pesticides | |||
| Hexachlorobenzene | 6.9 (3.9–15.4) | 8.2 (4.0–20.2) | 5.9 (3.3–13.6) |
| Oxychlordane | 6.7 (<LOD-52.1) | 6.6 (<L0D-15.8) | a |
| Trans-nonachlor | 16.6 (5.5–85.6) | 10.9 (2.8–29.9) | 3.5 (<LOD-9.6) |
| p,p’-DDD | a | a | a |
| -p,p’-DDE | 90.8 (51.4–1440) | 231 (29.7–3590) | 34.6 (15.9–230.0) |
| -o,p’-DDT | a | a | a |
| p,p’-DDT | a | a | a |
| Mirex | 6.9 (3.9–15.4) | 3.3 (<L0D-9.5) | a |
| Dioxins and furans (pg/g lipid) | |||
| 1,2,3,4,6,7,8,-Hepta CDD | 28.1 (4.8–92.9) | 10.9 (9.8–16.7) | 11.8 (7.5–21.6) |
| 1,2,3,4,6,7,8-Hepta CDF | 10.8 (32–21.6) | 6.9 (3.1–41.3) | 5.7 (3.4–20.6) |
| 1,2,3,4,7,8,9-Hepta CDF | a | a | 1.5 (0.7–2.9) |
| 1,2,3,4,7,8-Hexa CDD | 6.2 (<LOD-9.0) | 1.8 (<L0D-3.3) | 1.1 (0.5–1.4) |
| 1,2,3,4,7,8-Hexa CDF | 5.2 (<L0D-8.1) | 2.2 (<L0D-3.7) | 1.5 (0.6–2.8) |
| 1,2,3,6,7,8-Hexa CDD | 20.7 (<LOD-75.7) | 10.1 (3.8–17.8) | 5.9 (2.7–9.5) |
| 1,2,3,6,7,8-Hexa CDF | 6.2 (<L0D-9.0) | 32 (1.1–3.8) | 1.5 (0.6–2.8) |
| 1,2,3,7,8,9-Hexa CDD | 4.2 (<LOD-13.8) | 2.1 (<L0D-3.8) | 1.5 (0.8–2.2) |
| 1,2,3,7,8-Penta CDD | 4.4 (<L0D-19.1) | 3.1 (<L0D-4.3) | 1.8 (<L0D-3.3) |
| 2,3,4,7,8-Penta CDF | 7.1 (<L0D-10.6) | 3.6 (2.1–5.8) | 1.4 (0.8–5.6) |
| OCDD | 257.0 (59.5–1450) | 123 (88.2–201) | 98.8 (70.6–180) |
| OCDF | 6.2 (<LOD-22.6) | 1.6 (0.8–2.9) | 3.9 (3.3–10.3) |
| Dioxin-like PCBs (co-planar PCBs) (pg/g lipid) | |||
| PCB 77 (ng/g lipid) | a | a | a |
| PCB 81 (pg/g lipid) | 1.9 (1.7–3.5) | 2.8 (1.4–7.7) | a |
| PCB 126 (ng/g lipid) | 10.9 (<L0D-57.1) | 27.3 (6.4–95.4) | a |
| PCB 169 (pg/g lipid) | 16.5 (4.3–49.3) | 16.4 (6.5–52.2) | a |
| PFAS (μg/L)c | |||
| Et-PFOSA-AcOH | a | ||
| Me-PFOSA-AcOH | 0.5 (0.1–1.5) | ||
| PFDeA | 5.4 (1.3–10.2) | ||
| PFHxS | 4.4 (0.9–11.0) | ||
| PFNA | 11.6 (2.5–27.3) | ||
| PFOA | 6.8 (4.1–18.9) | ||
| PFOS | 88 (17.6–181) | ||
| PFOSA | a | ||
| PCBs | |||
| PCB 28 | a | 0.80 (<LOD-5.0) | 0.5 (<LOD-7.5) |
| PCB 44 | a | a | a |
| PCB 49 | a | a | a |
| PCB 52 | a | a | a |
| PCB 66 | 0.5 (<LOD-1.5) | 0.9 (<LOD-4.3) | a |
| PCB 74 | 3.3 (<L0D-18.0) | a | a |
| PCB 87 | a | a | a |
| PCB 99 | 2.1 (1.6–23.4) | 4.6 (1.9–16.1) | 0.9 (0.4–7.8) |
| PCB 101 | a | 0.5 (<LOD-4.9) | a |
| PCB 105 | 0.9 (<LOD-4.6) | 1.3 (0.5–4.6) | a |
| PCB 110 | a | a | a |
| PCB 118 | 4.5 (1.5–27.9) | 7.0 (2.6–252) | 1.7 (0.9–5.8) |
| PCB 128 | a | a | a |
| PCB 138 + 158 | 9.2 (5.7–73.4) | 17.4 (6.8–44.6) | 2.7 (1.1–54.3) |
| PCB 146 | 3.8 (1.2–19.5) | 5.20 (12–11.7) | a |
| PCB 149 | a | a | a |
| PCB 151 | a | a | a |
| PCB 153 | 23 (9.3–113.4) | 35.1 (13.1–81.9) | 3.6 (1.2–75.0) |
| PCB 154 | a | a | a |
| PCB 156 | 4.7 (0.9–16.1) | 4.7 (1.0–11.0) | a |
| PCB 157 | 1.2 (<L0D-4.2) | 1.0 (<L0D-2.3) | a |
| PCB 158 | With congener 138 | With congener 138 | With congener 138 |
| PCB 167 | 1.1 (<L0D - 5.8) | 0.8 (<L0D - 3.2) | a |
| PCB 170 | 9.3 (2.3–32.6) | 9.8 (3.1–26.9) | 0.6 (<LOD-20.5) |
| PCB 172 | 22 (0.4–6.9) | 1.5 (<L0D-4.2) | a |
| PCB 177 | 1.1 (0.7–4.2) | 1.8 (0.5–4.4) | a |
| PCB 178 | 42 (<L0D-14.6) | 2.2 (0.7–5.3) | a |
| PCB 180d | 32.8 (6.5–101.1) | 25.1 (82–69.3) | 1.6 (0.6–48.3) |
| PCB 183 | 2.4 (1.3–22.5) | 2.1 (1.1–6.9) | a |
| PCB 187d | 17.1 (5.4–62.0) | 9.8 (3.0–24.7) | 0.7 (<LOD-10.2) |
| PCB 189 | a | 0.6 (<L0D-1.9) | a |
| PCB 194d | 14.3 (<L0D-45.7) | 6.7 (1.7–18.3) | 0.5 (<LOD-6.5) |
| PCB 195 | 0.6 (<LOD-5.8) | 1.1 (0.6–3.1) | a |
| PCB 196d | 23.4 (5.9–95.1) | 5.0 (1.8–11.5) | 0.6 (<LOD-4.8) |
| PCB 199d | 37.5 (9.3–136.7) | 7.1 (1.8–15.9) | 0.5 (<LOD-3.3) |
| PCB 201d | a | a | a |
| PCB 202d | a | a | a |
| PCB 206d | 30.7 (6.6–112.7) | 3.8 (0.8–6.6) | 0.4 (<LOD-0.8) |
| PCB 207d | a | a | a |
| PCB 208d | a | a | a |
| PCB 209d | 8.1 (1.9–40.2) | 2.3 (0.5–5.4) | a |
| Sum of PCBse | 233 (74.5–882) | 157 (56.5–352) | 15.4 (4.2–270) |
| Sum of congeners comprising Aroclor 1268f | 134 (38.9–466) | 52.9 (16.5–127) | 4.2 (0.6–69.5) |
| Proportion of PCBs from Aroclor 1268 congeners | 52%(48%−68%) | 34%(19%−46%) | 24% (14%−32%) |
Analyte detected in fewer than 60% of specimens; no statistical analyses done.
<LOD = Less than the analytic limit of detection.
PFAS not analyzed in human specimens from Miami or Charleston Harbor.
PCB congeners present in Aroclor 1268 (Maruya, 1997; Schwope et al., 2005).
Sum of PCBs includes congeners 28, 44, 49, 52, 66, 74, 87, 99, 101, 105, 110, 188, 128, 146, 149, 151, 153, 156, 157, 167, 170, 172, 177, 178, 180, 183, 187, 189, 194, 195, 199, 206, 209, 138 + 158 when a congener was detected in at least 60% of the specimens.
Sum of PCB congeners comprising Aroclor1268 when a congener was detected in at least 60% of the specimens.
Table 3.
Contaminant concentrations (trace elements, PBDEs, chlorinated pesticides, dioxins, and PFAS) measured in Sapelo Island area resident male dolphins provided by Dr. Lori Schwacke (from Balmer et al., 2011); in northern Biscayne Bay, Miami area resident male and juvenile dolphins from Dr. Jenny Litz (Litz et al., 2007); and Charleston Harbor area resident dolphins provided by Dr. John Kucklick and given in Pat Fair et al. (2012). Values are median (range) in ng/g lipid from blubber unless otherwise noted.
| Analyte | Sapelo n = 37 | Miami n = 16 | Charlestonc n = 20 |
|---|---|---|---|
| PBDEs | |||
| PBDE 47 | 1640 (347–14,300) | 1380 (300–4510) | 3160 (226–40,300) |
| PBDE 99 | 85.0 (3.5–875) | 211 (45.1–338) | 291 (8.0–1830) |
| PBDE100 | 755 (115–7370) | 740 (91.9–1720) | 1030 (53–8190) |
| PBDE153 | 149 (60.0–2090) | 95.0 (28.0–264) | 209 (6–688) |
| PBDE154 | 251 (68.3–3510) | 161 (30.4–573) | 522 (71.4–1380) |
| Median sum of PBDE 47,99,100,153,154 | 2830 (774–26,900) | 2620 (496–6830) | 4900 (410–52,200) |
| Chlorinated pesticides | |||
| Hexachlorobenzene | 43.9 (10.7–419) | 39.0 (5.5–199) | 160 (78.2–320) |
| Oxychlordane | a | 590 (198–1500) | a |
| Trans-nonachlor | 3390 (948–51,300) | 5140 (570–17,500) | 7180 (937–29,600) |
| p,p’-DDD | With o,p’-DDT | With o,p’-DDT | With o,p’-DDT |
| p,p’-DDE | 18,800 (5990–199,000) | 13,800 (2790–31,500) | 29,200 (3110–86,700) |
| o,p’-DDT | With p,p’-DDD | With p,p’-DDD | With p,p’-DDD |
| p,p’-DDT | 130 (41.2–1560) | 186 (53.4–745) | 457 (203–1070) |
| o,p’-DDT+ p,p’-DDD | 1800 (762–24,300) | 1090 (107–3930) | 3590 (1820–7740) |
| Mirex | 2130 (589–17,100) | 159 (48.9–371) | 1170 (438–4970) |
| PFAS (ng/gwet mass) | |||
| PFDA | 38.9 (27.1–66.5) | a | 130 (48.1–368) |
| PFHxS | 18.2 (10.3–35.0) | a | 40.2 (7.6–126) |
| PFNA | 49.0 (35.4–105) | a | 63.2 (18.7–275) |
| PFOA | 18.7 (12.8–39.8) | a | 34.9 (8–275) |
| PFOS | 335 (235–478) | a | 1140 (395–2460) |
| PFOSA | 8.0 (6.1–13.0) | a | 25.4 (7.7–69.3) |
| Sum PFAS | 467 | a | 1430 |
| PCB congeners | |||
| PCB 28 | 29.3 (11.2–246) | 47.5 (13.3–114) | 21.5 (1.3–139) |
| PCB 44 | 24.0 (2.3–350) | 64.5 (9.0–371.5) | 70.7 (31.3–127) |
| PCB 49 | 63.6 (24.9–575) | 398 (23.7–2400) | 153 (31.4–2720) |
| PCB 52 | 876 (304–11,400) | 5230 (194–17,100) | 1840 (288–4100) |
| PCB 66 | 150 (68–1390) | 327 (54.5–900) | 242 (126–425) |
| PCB 74 | 150 (12–1250) | 374 (67.0–1260) | 274 (125–397) |
| PCB 87 | 29.9 (4.9–430) | 111 (11.8–595) | 62.1 (12–1400) |
| PCB 99 | 3570 (1230–63,000) | 16,700 (836–48,500) | 5500 (1200–15,400) |
| PCB 101 | 1200 (251–11,600) | 3370 (250–12,000) | 2020 (693–3360) |
| PCB 105 | 316 (1.8–1530) | 919 (136–2780) | 434 (205–792) |
| PCB 110 | 26.8 (12.8–311) | 122 (10.4–474) | 71.4 (4.7–1580) |
| PCB 118 | 1060 (313–11,900) | 4540 (658–12,800) | 1630 (729–3010) |
| PCB 128 | 813 (272–14,800) | 3474 (178.0–7996) | 1470 (215–4090) |
| PCB 138 | 6090 (1820–114,000) | 19,800 (1160–53,300) | 8710 (1800–28,600) |
| PCB 146 | 1690 (509–19,300) | 3790 (330–8570) | 2010 (463–3910) |
| PCB 149 | 3940 (1460–70,800) | 6290 (277–18,800) | 5400 (830–14,200) |
| PCB 151 | 1490 (421–24,700) | 2360 (107–7580) | 1740 (280–5670) |
| PCB 153 | 16,000 (5180–301,000) | 54,100 (1400–140,000) | 21,500 (4450–65,700) |
| PCB 154 | 1530 (530–29,300) | 1426 (90.8–3100) | 799 (148–2100) |
| PCB 156 | 90.1 (24.4–713) | 340 (52.2–1230) | 149 (22.3–293) |
| PCB 157 | 57.3 (23.4–679) | 243 (28.9–514) | 80.4 (23.3–144) |
| PCB 158 | 329 (91.0–6270) | 1190 (55.3–2920) | 713 (118–1770) |
| PCB 167 | 132 (36.8–1310) | 398 (60.1–1060) | 171 (52.5–265) |
| PCB 170 | 1710 (471–27,400) | 5330 (331–13,800) | 2230 (647–6860) |
| PCB 172 | 330 (107–4300) | 452 (43.7–1200) | 113 (38.0–378) |
| PCB 177 | 1220 (368–20,300) | 2040 (105–6200) | 1720 (365–5090) |
| PCB 178 | 2470 (878–38,000) | 1580 (97.3–3400) | 1380 (302–3900) |
| PCB 180b | 6340 (1840–116,000) | 11,300 (737–33,200) | 7240 (2380–23,100) |
| PCB 183 | 2930 (1000–65,800) | 3680.0 (274–10,300) | 2220 (597–6640) |
| PCB 187b | 12,600 (4340–277,000) | 11,300 (827–30,800) | 8000 (1990–22,100) |
| PCB 189 | 1420 (130–66,200) | 164 (8.5–362) | 77.8 (31.7–186) |
| PCB 194b | 10,400 (2110–301,000) | 2450 (270–7080) | 1830 (649–5090) |
| PCB 195 | 334 (130–5780) | 489 (50.8–1130) | 348 (116–788) |
| PCB 196b | 9310 (3320–207,100) | 2900 (296–7440) | 2700 (1060–4260) |
| PCB 199b | 16,000 (5080–362,000) | 3200 (269–8690) | 3740 (1160–9600) |
| PCB 201b | 2570 (878–55,500) | 601 (62.2–1380) | 715 (206–2010) |
| PCB 202b | 7270 (2580–178,000) | 1320 (138–3090) | 1770 (487–4910) |
| PCB 206b | 9240 (3680–191,000) | 961 (140–2720) | 1770 (724–4590) |
| PCB 207b | 1200(437–2390) | 156 (33.1–444) | 294 (138–881) |
| PCB 208b | 4990 (1920–106,000) | 487 (78.6–1370) | 1080 (408–3190) |
| PCB 209b | 1400 (464–25,700) | 95.2 (16.6–332) | 552 (225–1860) |
| Sum of PCBs | 133,000 (47,600–2,760,000) | 201,000 (12,600–484,000) | 95,700 (26,700–263,000) |
| Sum of congeners comprising Aroclor 1268 | 75,400 (29,700–1840,000) | 34,700 (2870–93,100) | 29,600 (10,000–79,600) |
| Proportion of PCBs from Aroclor 1268 congeners | 60% (49%−67%) | 20% (15%−24%) | 30% (19%−38%) |
Not analyzed in Miami dolphins.
Major congener in Aroclor 1268.
PFAS values given in Pat Fair et al. (2012) are geometric means (95% confidence intervals).
Table 4.
Comparison of contaminant concentrations (trace elements, PBDEs, chlorinated pesticides, dioxins, and PFASs) measured in seafood provided by study participants. Values are ng/g lipid unless otherwise specified.
| Analyte | Sapelo fish filets (n = 8) Median (range) | Miami fish filets (n = 17) Median (range) | Charleston seafooda (n = 8) Median (range) |
|---|---|---|---|
| Trace elements (mg/kg) | |||
| Cadmium | b | c | b |
| Lead | b | c | b |
| Mercury | 0.05 (0.03–0.09) | 0.2 (0.008–0.3) | b |
| Selenium | 0.5 (0.4–0.6) | 0.4 (0.3–1.5) | b |
| PBDEs | |||
| PBDE 47 | 12.6 (<LOD-29.6) | b | b |
| PBDE 99 | b | b | b |
| PBDE 100 | b | 3.0 (<LOD-5.4) | b |
| PBDE 153 | 4.2 (<LOD-8.5) | 13.3 (<LOD-64.6) | b |
| PBDE 154 | b | 13.6 (<LOD-65.7) | b |
| Median sum of PBDE 47, 99,100,153,154 | 16.4 (6.9–33.0) | 23.6 (<LOD-130) | b |
| Chlorinated pesticides | |||
| Hexachlorobenzene | b | c | b |
| Oxychlordane | b | b | b |
| Trans-nonachlor | b | b | b |
| p,p’-DDD | 57.3 (17.8–143) | 11.6 (<LOD-18.4) | b |
| p,p’-DDE | 132 (28.1–494) | 93.2 (<LOD-148) | b |
| p,p’-DDT | 42.2 (10.2–114) | b | b |
| 4,4’-DDTp,p’-DDT | 67.3 (19.6–146) | 4.43 (<LOD-10.7) | b |
| Mirex | 31.5 (5.26–189) | b | b |
| Dioxins and furans (pg/g lipid) | |||
| 1,2,3,4,6,7,8,-Hepta CDD | b | 0.3 (0.2–0.9) | b |
| 1,2,3,4,6,7,8-Hepta CDF | 0.2 (<LOD-0.4) | b | b |
| 1,2,3,4,7,8,9-Hepta CDF | b | c | b |
| 1,2,3,4,7,8-Hexa CDD | b | 0.02 (<LOD-0.04) | b |
| 1,2,3,4,7,8-Hexa CDF | b | b | b |
| 1,2,3,6,7,8-Hexa CDD | 0.2 (<LOD-0.5) | 0.3 (0.2–0.8) | b |
| 1,2,3,6,7,8-Hexa CDF | b | 0.02 (<LOD-0.04) | b |
| 1,2,3,7,8,9-Hexa CDD | 0.1 (<LOD-0.3) | 0.2 (<LOD-0.7) | b |
| 1,2,3,7,8-Penta CDD | b | b | b |
| 1,2,3,7,8-Penta CDF | b | b | b |
| OCDD | 1.5 (<LOD-5.2) | 0.8 (0.3–3.7) | b |
| OCDF | c | c | b |
| Dioxin-like PCBs (coplanar PCBs) | |||
| 334455P (PCB 169) | c | c | b |
| 33445P (PCB 126) lipid) | c | c | b |
| 3344P (PCB 77) | c | c | b |
| 3445P (pCB 81) | c | c | b |
| PCB congeners | |||
| PCB 28 | b | b | |
| PCB 44 | b | b | |
| PCB 49 | b | 25.3 (<LOD-61.2) | b |
| PCB 52 | b | 17.5 (<LOD-40.4) | b |
| PCB 66 | b | 11.5 (<LOD-21.3) | b |
| PCB 74 | b | 5.19 (<LOD-13.1) | b |
| PCB 87 | b | b | b |
| PCB 99 | b | b | b |
| PCB 101 | b | b | b |
| PCB 105 | b | b | b |
| PCB 110 | b | b | b |
| PCB 118 | b | 76.8 (<LOD-109.3) | b |
| PCB 128 | b | 13.2 (<LOD-31.7) | b |
| PCB 138 | b | 78.2 (51.3–176) | b |
| PCB 146 | 15.0 (<LOD-35.1) | 29.0 (<LOD-63.0) | b |
| PCB 149 | b | 18.9 (<LOD-35.0) | b |
| PCB 151 | b | b | b |
| PCB 153 | 97.2 (<LOD-158) | 221 (<LOD-410.3) | b |
| PCB 154 | 5.21 (<LOD-18.1) | 10.0 (<LOD-15.3) | b |
| PCB 156 | b | 6.19 (<LOD-16.3) | b |
| PCB 157 | b | b | |
| PCB 158 | 25.6 (7.27–54.1) | 4.21 (<LOD-7.79) | b |
| PCB 167 | b | 4.3 (<LOD-9.86) | b |
| PCB 170 | b | 21.3 (<LOD-43.6) | b |
| PCB 172 | b | 3.89 (<LOD-8.67) | b |
| PCB 177 | b | 5.68 (<LOD-19.5) | b |
| PCB 178 | 9.6 (<LOD-30.2) | 3.54 (<LOD-16.5) | b |
| PCB 180d | b | b | b |
| PCB 183 | b | 13.8 (<LOD-32.7) | b |
| PCB 187d | b | b | b |
| PCB 189 | b | b | b |
| PCB 194d | b | 12.1 (<LOD-25.7) | b |
| PCB 195 | b | b | b |
| PCB 196d | 33.6 (8.22–176) | 15.2 (<LOD-24.4) | b |
| PCB 199d | 45.1 (11.9–240) | 7.64 (<LOD-21.8) | b |
| PCB 201d | 7.26 (1.75–38.7) | 1.99 (<LOD-4.48) | b |
| PCB 202d | 21.7 (5.28–111) | 2.24 (<LOD-10.6) | b |
| PCB 206d | 58.1 (10.7–246) | 6.15 (<LOD-13.1) | b |
| PCB 207d | b | b | b |
| PCB 208d | 23.8 (5.36–96.9) | b | b |
| PCB 209d | 15.7 (3.33–58.9) | 1.87 (<LOD-2.79) | b |
| Sum of PCBs | 353 (77.7–1440) | 632 (3.95–1650) | b |
| Sum of congeners comprising Aroclor 1268 | 240 (53.6–1100) | 66.4 (<LOD-324) | b |
| Proportion of PCBs from Aroclor 1268 congeners | 71% (48.6%−82.7%) | 8.29% (0%−19.6%) | b |
Fish samples comprised shrimp and crabs.
Analyte detected in less than 60% of the samples.
Not analyzed in seafood provided by study participants.
PCB congeners present in Aroclor 1268 (Maruya, 1997; Schwope et al., 2005).
3.2.1. Metals and selenium
We detected cadmium, lead, mercury and selenium in all human blood specimens (Table 2). Blood specimens from Miami had the highest levels of lead, mercury (above NHANES Survey 2009–2010 95th percentile) and cadmium (above NHANES Survey 2009–2010 50th percentile). Sapelo Island blood specimens had median concentrations of cadmium, lead, and mercury between the 50th and 95th percentiles of the comparison group in NHANES 2009–2010. Blood specimens from Charleston had the lowest levels of the metals listed above but the highest selenium concentrations. Mercury and selenium were found in fish samples from Sapelo Island and Miami; the levels of mercury in fish from Miami were four times higher than those found in fish from Sapelo Island (0.2 mg/kg versus 0.05 mg/g, respectively, Table 4).
3.2.2. Brominated flame retardants
For PBDEs, we provided individual PBDEs and the median sums of PBDEs 47, 99, 100, 153, and 154 (the congeners most commonly found in the environment [DeWit, 2002]), for people, dolphins, and fish (Tables 2, 3, and 4, respectively). PBDEs were detected in blood from all three human study populations (Table 2). The median concentrations for most congeners were below the 50th percentile of the respective NHANES comparison group. However, the median levels of PBDEs 99 and 153 were between the 50th and 95th percentiles of NHANES comparison categories in the three study populations and the level of PBDE 183 in Miami study participants was above the NHANES 95th percentile. The presence and concentrations of PBDEs in dolphins varied across the populations; however, all blubber specimens contained measurable concentrations of PBDEs (Table 3). PBDE congeners 100, 153, and 154 were detected in more than 60% of fish samples from Miami whereas only PBDEs 47 and 153 were detected in more than 60% of fish samples from Sapelo Island.
3.2.3. Chlorinated pesticides
Many of the chlorinated pesticides we examined were present in human serum at median concentrations less than the 50th percentile for the NHANES comparison group (Table 2, Supplemental Table 2). Some of the compounds were above the NHANES 2003–2004 50th percentile in Sapelo and/or Miami, including oxychlordane, trans-nonachlor, and mirex. The types and quantities of chlorinated pesticides varied across dolphin populations (Table 3). DDT and metabolites were detected in fish samples (Table 4) from Sapelo Island and Miami.
3.2.4. Dioxins
The median concentrations of nearly all the dioxins and furans measured in study participants were above either the 50th percentiles or the 95th percentiles for their respective NHANES comparison groups (Table 2). In particular, the median concentrations of 1,2,3,7,8,9-Hexa CDD and OCDF were above the 95th percentiles of their NHANES comparison groups for all areas.
3.2.5. PFAS
Six FAS compounds were detected in all serum specimens from Sapelo Island (Table 2). Of these, the median concentrations of four (PFDeA, PFNA, PFOA, and PFOS) were well above the NHANES 95th percentile; and the median concentrations of Me-PFOSA-AcOH and PFHxS were between the NHANES 50th and 95th percentiles for males. We did not test blood specimens from people in Miami or Charleston Harbor for PFAS. PFAS were also measured in dolphin plasma from animals sampled near Sapelo Island and Charleston Harbor (Table 3). The sum of PFASs was three fold higher in Charleston Harbor dolphin plasma than in plasma from dolphins near Sapelo Island (1430 ng/g versus 467 ng/g) and was largely driven by higher PFOS concentrations (1140 ng/g vs 335 ng/g; Table 3).
3.2.6. PCBs
Study participants from Sapelo Island and Miami had levels of most of the dioxin-like PCBs greater than the 50th percentile of the respective NHANES comparison groups. Dolphins and fish samples were not analyzed for dioxin-like PCBs.
For the non-dioxin-like PCB congeners known to be part of Aroclor 1268 (PCBs 180, 187, 194, 196, 199, 201, 202, 206, 207, 208, and 209 comprising 0.6%, 0.9%, 1.8%, 2.7%, 3.0%, 3.0%, 7.1%, 9.1%, 9.9%, 11%, 50% of congeners in that mixture, respectively) (Maruya, 1997; Schwope et al., 2005) the lipid-adjusted median concentrations tended to be higher in fish than in people (Tables 4 and 2, respectively). The highest concentrations of these Aroclor 1268 PCB congeners were found in dolphins (Table 3). This suite of congeners comprised twice the proportion of PCB congeners in Sapelo Island dolphins (60%) versus those sampled from Charleston, SC (30%) and Miami, FL (20%) (Table 3). Aroclor 1268 congeners represented 52%, 24%, and 34% of the total PCBs present in human specimens from Sapelo Island, Charleston Harbor, and Miami, respectively (Table 2). These congeners represented a much higher proportion of total PCBs in fish from Sapelo Island than in fish from Miami (71% and 8.29%, respectively).
The sum of non-dioxin-like PCB congeners was highest in dolphins from Miami, followed by those from Sapelo Island and then Charleston Harbor (median 201,000 ng/g lipid, 133,000 ng/g lipid, and 95,700 ng/g lipid, respectively). In human serum specimens from Sapelo Island, the median PCB concentrations were greater than the NHANES comparison group 95th percentiles for three congeners (196, 199, 206) and were between the NHANES comparison group 50th and 95th percentiles for 11 additional congeners (146, 157, 167, 170, 172, 178, 180, 183, 187, 194, and 209) (Table 2). In serum specimens from Miami, median PCB concentrations were greater than the NHANES comparison group 50th percentile for 22 congeners (99, 105, 118, 138 + 158, 146, 153, 156, 157, 167, 170, 172, 177, 178, 180, 183, 187, 189, 194, 195, 196, 199, and 206). Study participants from Charleston had the lowest PCB levels with all detected congener concentrations below the NHANES 50th percentile.
For PCBs that were analyzed in both human serum specimens and fish (Tables 2 and 4, respectively), the percent detected in each type of sample varied with the individual PCB congener. For example, PCB 28 was detected in human serum specimens from Miami and Charleston Harbor, but not in those from Sapelo Island. PCB 74 was detected in fish samples from Miami, but not in fish samples from Sapelo Island.
The Sapelo Island community is near a former production site for the PCB mixture Aroclor 1268. PCB congeners known to be part of Aroclor 1268 are: PCBs 180, 187, 194, 196, 199, 201, 202, 206, 207, 208, and 209 comprising 0.6%, 0.9%, 1.8%, 2.7%, 3.0%, 3.0%, 7.1%, 9.1%, 9.9%, 11%, 50% of congeners in that mixture, respectively (Maruya, 1997; Schwope et al., 2005). Figs. 1–3 are examples of how study participant data compared with NHANES (Survey 2003, 2004 results).
Fig. 1.
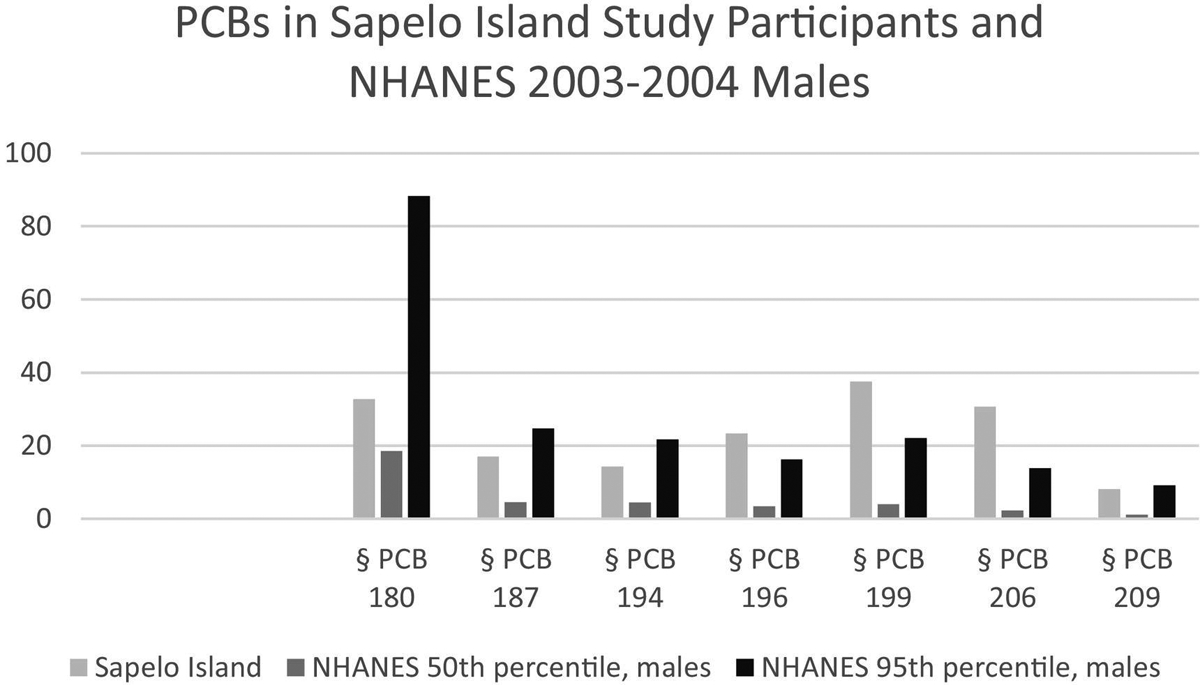
Serum concentrations of non-dioxin-like PCBs comprising Aroclor 1268 that were analyzed in study participants. Comparison values from NHANES 2003–2004 (CDC, 2018) are in Supplemental Table 2; comparison values are from NHANES males.
Fig. 3.
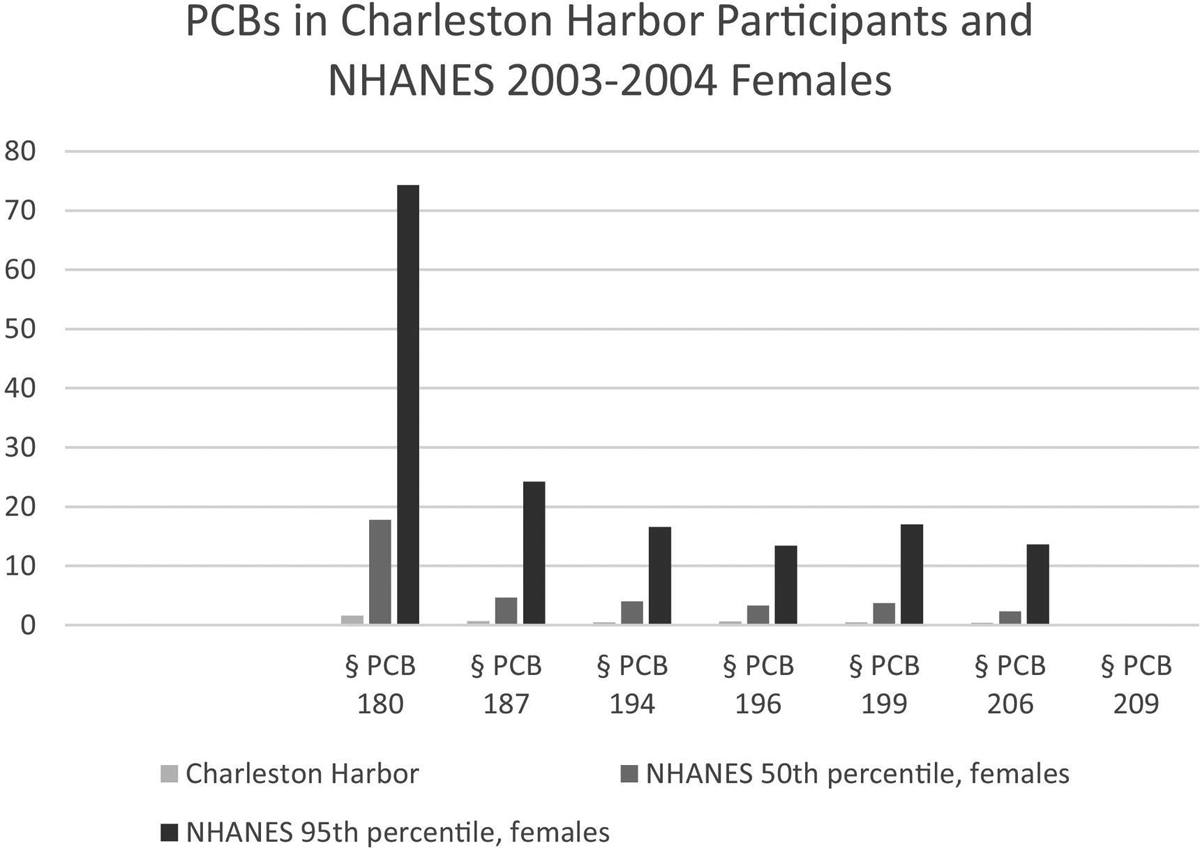
Serum concentrations of non-dioxin-like PCBs comprising Aroclor 1268 that were analyzed in study participants. Comparison values from NHANES 2003–2004 (CDC, 2018) are in Supplemental Table 2; comparison values are from NHANES females. Values are ng/g lipid.
Comparisons of the Log10(minimums, medians, and maximums) for the contaminants found in at least 60% of both human specimens and dolphin samples are presented in Figs. 4–6 for Sapelo Island, Miami, and Charleston Harbor, respectively. In general, the analytes that were higher in dolphins were also higher in people.
Fig. 4.
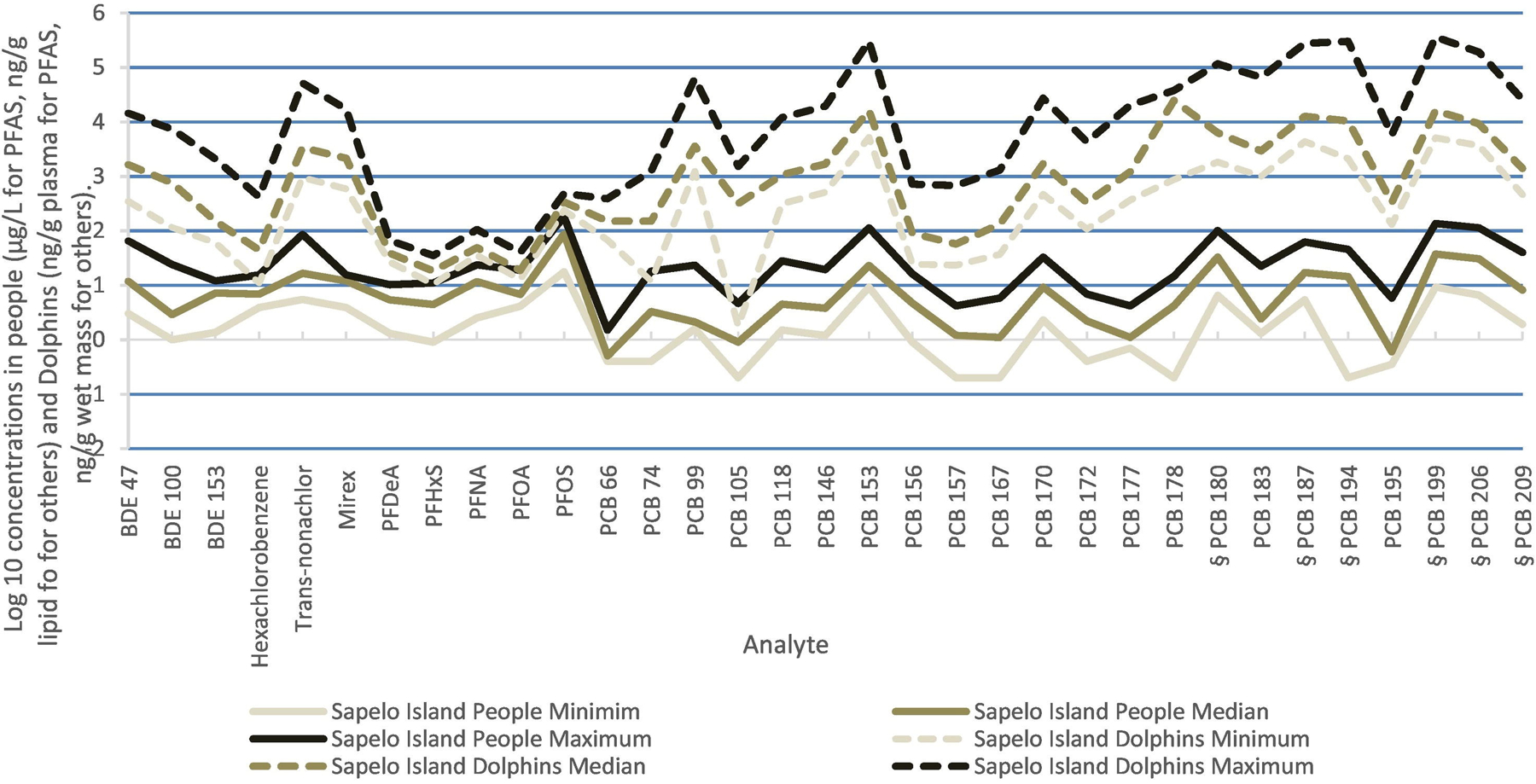
Contaminants in Sapelo Island people (μg/L for PFAS, ng/g lipid for others) and Dolphins (ng/g Lipid). Analytes are those detected in at least 60% of samples from both people and dolphins. Values are Log10(minimum, median, maximum). For minimum values below the analytical LOD, we substituted (LOD × 0.5).
Fig. 6.
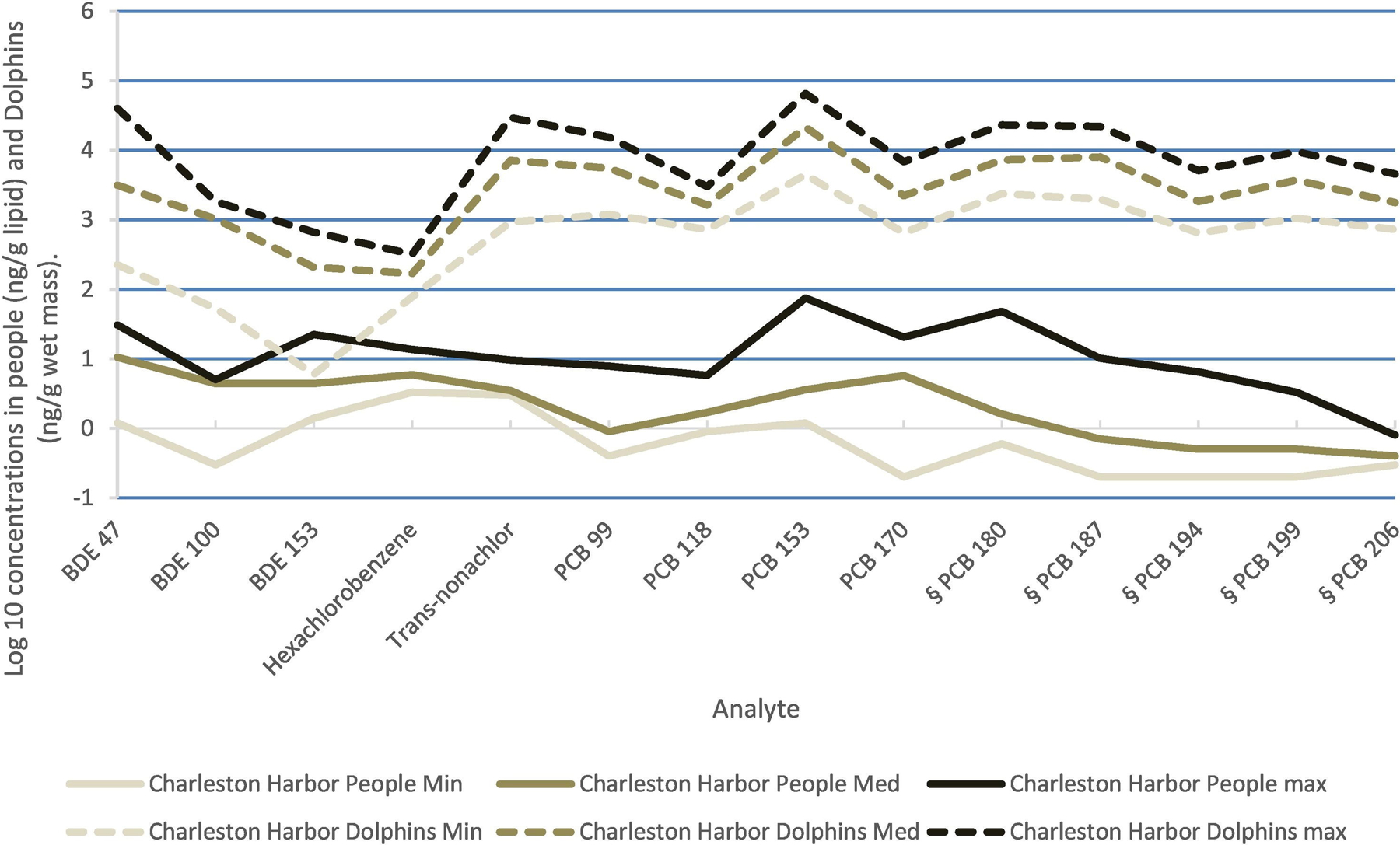
Contaminants in Charleston Harbor people (μg/L for PFAS, ng/g lipid for others) and Dolphins (ng/g Lipid). Analytes are those detected in at least 60% of samples from both people and dolphins. Values are Log10 (minimum, median, and maximum). For minimum values below the analytical LOD, we substituted (LOD × 0.5).
4. Discussion
4.1. Metals and selenium
The occupations reported by study participants (e.g., boat crew, teacher; data not shown) were not those typically associated with exposure to metals measured in this study; however, we detected cadmium, lead, mercury, and selenium in blood specimens from all study participants, often at levels above the NHANES comparison group 50th or 95th percentiles (see Table 2). The higher concentrations of cadmium in some participants may reflect smoking-related exposures as nearly half (44%) of participants from Sapelo Island and nearly all (90%) participants from Miami reported current smoking. Mercury and selenium were also found in nearly all fish samples from Sapelo Island and Miami, indicating that locally-caught seafood is one source of ongoing exposures for these communities. The concentrations of lead and mercury in blood specimens was particularly high, i.e., above the 95th percentile for the NHANES comparison group in human samples from Miami (for lead: median, range in ug/L, 8.96; 2.35–35.0 and for mercury: 6.26; 2.22–40.1) and may reflect ongoing exposure from their food, fishing gear and/or other environmental sources.
4.2. PBDEs
PBDEs are a class of brominated flame retardants widely used in consumer products. U.S. manufacturers phased out PBDE congener production between 2004 and 2013 (EPA, 2017); however, these chemicals remain ubiquitous in the environment and have been detected in wildlife (DeWit, 2002; Muir et al., 2006; Hoguet et al., 2013) and in every person tested (Hites, 2004; Sjödin et al., 2003). In addition to diet, human exposure sources include house dust (Sjödin et al., 2003).
Regarding locational differences in PBDEs, Fair et al. (2007) reported concentrations of PBDEs in over 100 dolphins from the Indian River Lagoon and Charleston Harbor estuary. The sum of five PBDE congeners (lipid based) in male bottlenose dolphins (n = 31) sampled in the vicinity of Charleston Harbor was 6830 ng/g ± 2, 970 ng/g (mean ± SD) (Fair et al., 2007) compared to a median value of 4900 ng/g (range 410 ng/g to 52,200 ng/g) from Balmer et al., 2011 (Table 3). The sum of PBDEs in our study was similar to that reported by Fair et al. (2007), supporting the overall conclusion that PBDE concentrations are higher in Charleston Harbor dolphins than in the other locations studied. PBDE concentrations were considerably higher in dolphins from Charleston than in those from Miami. By contrast, total PBDE concentrations in people from Miami tended to be higher than in people from Charleston, suggesting an alternate source of environmental exposure. This exposure could be affected by how much time people spend indoors in areas full of consumer products containing PBDEs. Frederiksen et al. (2009) reported higher concentrations of PBDEs in indoor air and dust in North America than in Europe and Asia, possibly a result of different fire safety standards. The literature and our study results suggest that a portion of human exposure to PBDEs is through diet; however, the most important source of these chemicals is household dust and indoor air contaminated with flame retardants used in consumer products, such as upholstery (Frederiksen et al., 2009).
Sums of lipid-normalized PBDE congener concentrations were of the same magnitude in human blood and fish (Tables 2 and 4) while concentrations in dolphin blubber were approximately two orders of magnitude greater (Table 3). Lipid-normalized POPs concentrations in dolphin blubber are approximately the same as lipid-normalized concentrations in dolphin blood (Yordy et al., 2010). The finding that concentrations of the PBDEs measured in dolphins are much greater than those in people suggests that, in general, the routes of exposure to these PBDEs may be different between humans and dolphins. This finding also indicates that a seafood-dominated diet, which is the case for dolphins and for some human populations, is a major route of exposure to PBDEs.
4.3. Chlorinated pesticides
Although the organochlorines we analyzed were previously banned in the U.S., they are in limited use elsewhere (ATSDR, 2002). In general, organochlorine pesticide concentrations in serum are decreasing both in frequency of detection and in concentrations from year to year in the U.S. population (see CDC, 2018). However, our results suggest that regional pesticide use may have affected the detection rates and concentrations. Here, we focus on the results for 3 pesticides and metabolites that were commonly used in the Southeast: 1) p,p′-DDE a metabolite of DDT, which was used for mosquito and insect control on crops and inside buildings; 2) mirex, which was used to control imported fire ants and on corn and citrus; and 3) trans-nanochlor, the primary metabolite of chlordane, which was used for home termite control (National Pesticide Information Center, 1999; Buha, 2011; National Pesticide Information Center, 2001).
p,p′-DDE, a breakdown product of p,p′-DDT, is more persistent in the body than p,p′-DDT itself and so is thought to reflect historical exposure. Interestingly, despite very high p,p′-DDE concentrations in all the dolphin populations and the presence of p,p′-DDE in fish, the p,p′-DDE concentrations in human serum samples were generally lower across these three areas when compared with national NHANES 2003–2004 values. The medians for Sapelo Island and Charleston human serum specimens (90.8 ng/g lipid, 34.6 ng/g lipid, respectively) were well below the NHANES 50th percentiles for males and females (200 ng/g lipid and 207 ng/g lipid, respectively). The median for Miami human serum specimens (231 ng/g lipid) was higher than the NHANES 2003–2004 50th percentile (203 ng/g lipid), possibly reflecting the high concentrations of p,p′DDE in Miami fish (93.2 ng/g lipid) or other lifestyle-associated exposure sources.
The median level of mirex was highest in Sapelo Island human serum specimens followed by Miami (6.9 ng/g lipid and 3.3 ng/g lipid, respectively) and both were above the 50th percentile for the NHANES comparison group (<LOD). The median concentration of mirex in resident dolphins was an order of magnitude higher for animals collected near Sapelo Island (2130 ng/g lipid) than from near Miami (159 ng/g lipid) and mirex was detected in 100% of the fish samples from Sapelo Island, suggesting mirex was used more extensively in and around Sapelo Island. Mirex was heavily used in the Southeast until it was banned in 1978 (Huckins et al., 1982). We did not detect mirex in blood specimens from Charleston Harbor participants. By contrast, dolphins from that area had high levels of mirex (median 1170 ng/g lipid).
Trans-nonachlor levels were highest in people from Sapelo Island (median 16.6 ng/g lipid), whereas the median trans-nonachlor concentration was highest in dolphins from Charleston Harbor (median 7180 ng/g lipid). Our results for mirex and trans-nonachlor may reflect historic rather than current use of some pesticides in that part of the southeastern United States. Information about specific pesticide use (types, brands, amounts, etc.) would help determine what was in the local environment during the 1950s–1970s and might help explain the differences in contaminant concentrations between species and populations.
4.4. Dioxins
We detected dioxins in all samples from Sapelo Island and Charleston Harbor. This finding is likely associated with the historic prevalence of the paper industry in the southeast. Concentrations of dioxins in human serum specimens tended to be one to two orders of magnitude higher than concentrations in fish filets; thus, local fish are a source, though likely not the only source, of dioxin exposure for these population.
4.5. PFAS
PFAS are distributed throughout the global environment, leading to extensive exposure of people and wildlife and concerns about associated health effects (Fromme et al., 2009; Steenland et al., 2010; Vestergren and Cousins, 2009). The concentrations of four PFAS (PFDeA, PFNA, PFOA, PFOS) in Sapelo Island serum specimens were greater than the 95th percentile from NHANES (NHANES 2009–2010, males). For PFOS, the median for Sapelo Island serum specimens was 88 μg/L, twice the NHANES comparison group 95th percentile (37.4 μg/L). The median level of PFOS in Sapelo Island participants was also greater than the 95th percentile for NHANES 1999–2000 for non-Hispanic blacks (Kato et al., 2011a). For humans, we do not know the source of exposure to PFAS in the Sapelo community. Kato et al. (2011b) found that PFAS levels in NHANES varied by sociodemographics, including age, race, ethnicity, and sex. Environmental sources of exposure include carpets and kitchenware that have leached these chemicals into household air or food over time. Many sites in the United States have reported contamination of drinking water from use of firefighting foams containing PFAS (ASTDR, 2016; EPA, 2016).
Giesy and Kannan (2001) and Houde et al. (2006) found that PFAS were widely distributed in birds, fish, and mammals, with the highest concentrations in apex predators, including eagles, polar bears, and marine mammals. PFAS measurements in plasma from Charleston Harbor and Sapelo Island dolphins were also high, suggesting that these contaminants were widespread in the environment and could accumulate in all apex predators, including people, through food. Interestingly, the median concentration of PFOS in Sapelo dolphin plasma was considerably less than the geometric mean (Inv 3098 ng/g wet weight) reported for Chareston Harbor dolphins by Fair et al. (2012), suggesting that people in Charleston with high seafood intake may also be expected to have elevated PFOS levels.
4.6. PCBs
The PCB congeners that we found in people and fish were found in dolphins at high concentrations. For example, we detected PCB congeners 146 and 153 in Sapelo Island serum specimens and in fish filets. The same congeners were detected at high concentrations in resident dolphins (median concentrations: 1690 and 16,000 μg/g lipid, respectively), suggesting a common exposure source, likely the food web. The median sum of PCBs in Sapelo Island dolphins (133,000 ng/g lipid) reported in this study is similar to the levels of 10,000 to 761,000 μg/g lipid in blubber samples reported by Schwacke et al. (2012) in another study of male dolphins from Sapelo Island. These levels were associated with increased risk for anemia, hypothyroidism, and immune suppression in the dolphins.
Of particular interest were the more highly chlorinated PCB congeners detected in Sapelo Island people, dolphins, and fish. We detected a suite of congeners comprising a PCB mixture called Aroclor 1268 (Maruya, 1997; Ishikawa et al., 2005, and Schwope et al., 2005). Aroclor 1268 was produced and used at a manufacturing facility in Brunswick, Georgia, U.S. As a result of on-site contamination that threatened the nearby environment, the facility was named a Superfund site (Maruya and Lee, 1998; Kannan et al., 1997). Wirth et al. (2014) detected the Aroclor 1268 pattern of congeners in sediment and fish from the Sapelo Island area.
PCB congeners found in Sapelo Island dolphins, humans, and their seafood included congeners 196, 199, and 206, all components of Aroclor 1268. The median concentrations of these congeners in fish were of the same order of magnitude as we found in human serum; however, the concentrations in dolphins were up to three orders of magnitude higher. This makes sense considering the relative contributions of seafood to the diets of people compared with that of dolphins. These same four PCB congeners were present in Sapelo Island human serum specimens at concentrations above the 95th percentile. Finally, as noted above, PCB congeners associated with Aroclor 1268 represented 50% to 60% of total PCBs in humans and dolphins in Sapelo Island compared to 20% to 35% in humans and dolphins in Miami and Charleston Harbor. This difference demonstrates that local sources of contamination, i.e., a nearby Superfund site known to be contaminated with Aroclor 1268, are of the greatest concern both for coastal wildlife and for people.
Blood specimens from participants in Miami showed different patterns of PCB congeners than those from participants in Sapelo Island or Charleston Harbor. For example, Miami participants had higher concentrations of PCB 153 and much lower concentrations of the Aroclor 1268 congeners, a pattern more consistent with NHANES. However, Miami participants’ blood contained twenty-two PCB congeners present at median levels above the NHANES comparison group 50th percentile. As noted earlier, the median blood lead level was nearly 9 μg/dL in our study participants from Miami, well over both the NHANES comparison group 95th percentile and the reference blood level (5 μg/dL) specified by NIOSH (https://www.cdc.gov/niosh/topics/ables/description.htm). These participants were subsistence fishers and locally-caught seafood was a critical part of their diets. This group of subsistence fishers is particularly vulnerable to bioaccumulating local contaminants, particularly those in their food supply.
By comparing concentrations of POPs in human blood specimens with those from NHANES, we found that some populations are more highly exposed to specific suites or congeners of environmental contaminants than the U.S. population overall and that some of the exposures are site-specific. In general, the POPs that were higher in dolphins were also higher in people. Our results suggest that the presence and concentrations of pollutants in resident marine mammals can be useful in predicting which pollutants of concern should be further studied in human populations who share that environment, especially when human data are not available or would be difficult to obtain. Similarly, data from human biomonitoring research involving coastal communities may be useful in indicating the potential presence and relative levels of pollutants in marine animals, including protected species or those difficult to capture and evaluate. Finally, we found that a number of contaminants in our study were detected in fewer than 60% of the seafood samples from each area; and no contaminants were detected in at least 60% of the seafood provided by study participants in Charleston. Thus, although analyzing seafood may help assess the types of contaminants found at high levels in a given environment, we found that these data are less useful in assessing human exposure than the results from the dolphin studies were.
5. Conclusion
The patterns of environmental contaminants observed in human blood specimens, dolphin blood and blubber samples, and fish samples from three specific coastal communities reflect the types and quantities of pollutants in their respective environments. Data describing the types and amounts of environmental contaminants found in one coastal population (e.g. people) can be used to identify environmental pollutants of potential concern for other coastal populations of similar trophic level (e.g., dolphins) and thus guide future exposure and health outcome studies. It would be useful to more fully examine these and additional coastal communities to determine how living on the coast affects risks for exposure to specific environmental contaminants and associated adverse health outcomes compared to risks faced by non-coastal communities.
Supplementary Material
Fig. 2.
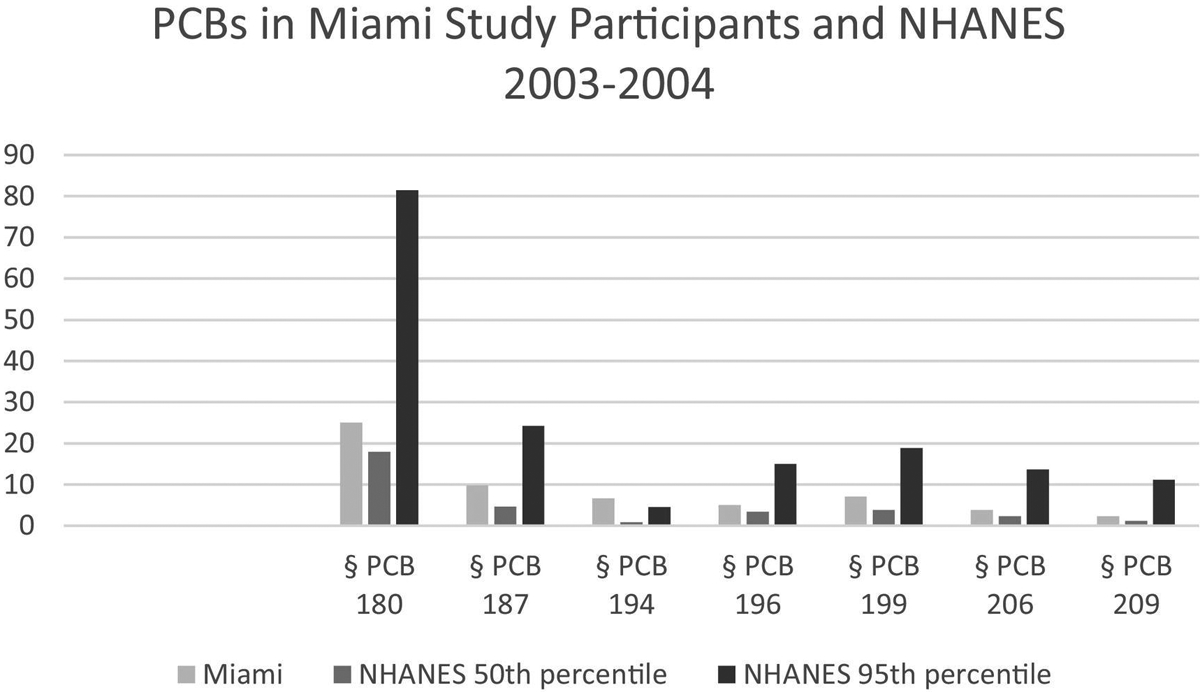
Serum concentrations of non-dioxin-like PCBs comprising Aroclor 1268 that were analyzed in study participants. Comparison values from NHANES 2003–2004 (CDC, 2018) are in Supplemental Table 2. Values are ng/g lipid.
Fig. 5.
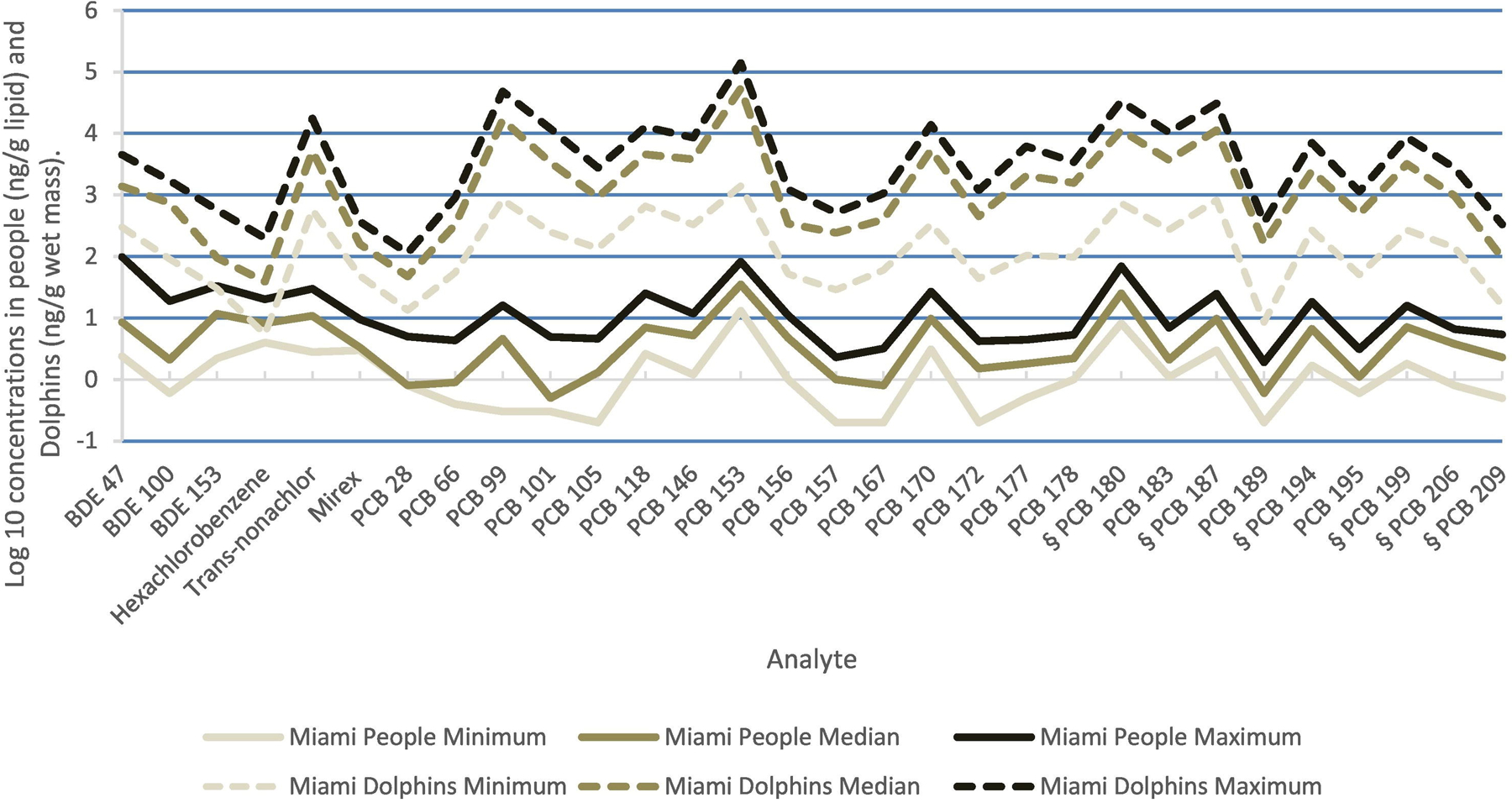
Contaminants in Miami people (μg/L for PFAS, ng/g lipid for others) and Dolphins (ng/g Lipid). Analytes are those detected in at least 60% of samples from both people and dolphins. Values are Log10(median). For minimum values below the analytical LOD, we substituted (LOD × 0.5).
HIGHLIGHTS.
Levels and proportions of specific POPs found in people and animals reflect POPs found in the local environment
Animal populations can be indicators of environmental pollutants of concern to coastal human populations
Humans may be indicators of pollutants of concern to nearby animal populations, including protected species.
Acknowledgements
The authors would like to thank Dr. Lori Schwacke, National Oceanic and Atmospheric Administration, for the data on Sapelo Island dolphins; Dorset Hurley, Georgia Department of Natura Resources, Wildlife Resources Division, Sapelo Island National Estuarine Research Reserve (SINERR), Sapelo Island GA; Dr. Kathleen Caldwell, Dr. Antonia Calafat, Dr. Robert Jones, and Dr. Andres Sjodin, Division of Laboratory Sciences, National Center for Environmental Health for the analysis of human blood specimens; Dr. Lora Fleming, University of Miami, European Centre for Environment and Human Health; Laura Aichinger-Dias, National Marine Fisheries Service, Southeast Fisheries Science Center; and Dr. Donna Johnson, Medical College of South Carolina, for their assistance in recruiting study participants; Dr. Helen Rogers and Johnni Daniel, National Center for Environmental Health, for their assistance with specimen collection, and Jessica Reiner for the National Institute of Standards and Technology for providing data on perfluorinated contaminants in dolphin blood.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Nomenclature1
Abbreviations
- DDD
Dichlorodiphenyldichloroethane
- DDE
Dichlorodiphenyldichloroethylene
- DDT
Dichlorodiphenyltrichloroethane
- PFAS
Per- and polyfluoroalkyl substances
- Et-PFOSA-AcOH
2-(N-ethyl-perfluorooctane sulfonamido) acetic acid
- Me-PFOSA-AcOH
2-(N-methyl-perfluorooctane sulfonamido) acetic acid
- PFDeA
Perfluorodecanoic acid
- PFHxS
Perfluorohexane sulfonic acid
- PFNA
Perfluorononanoic acid
- PFOA
Perfluorooctanoic acid
- PFOS
Perfluorooctane sulfonic acid
- PFOSA
Perfluorooctane sulfonamide
Polychlorinated dibenzo-p-dioxins and furans
- OCDD
1,2,3,4,6,7,8,9-Octachlorodibenzo-p-dioxin
- 1234678-Hepta CDD
1,2,3,4,6,7,8-Heptachlorodibenzo-p-dioxin
- 1234789-Hepta CDD
1,2,3,4,5,8,9-Heptachlorodibenzo-p-dioxin
- 123678-Hexa CDD
1,2,3,6,7,8-Hexachlorodibenzo-p-dioxin
- 123789-Hexa CDD
1,2,3,7,8,9-Hexachlorodibenzo-p-dioxin
- 12378-Penta CDD
1,2,3,7,8-Pentachlorodibenzo-p-dioxin
- OCDF
Octachlorodibenzofuran
- 1234678-Hepta CDF
1,2,3,4,6,7,8-Heptachlorodibenzofuran
- 123478F Hexa CDF
1,2,3,4,7,8-Hexachlorodibenzofuran
- 123678-Hexa CDF
1,2,3,6,7,8-Hexachlorodibenzofuran
- 12378-Penta CDF
1,2,3,7,8-Pentachlorodibenzofuran
Polybrominated diphenyl ethers
- BDE 17
2,2′,4′-Tribromodiphenyl ether
- BDE 28
2,4,4′-Tribromodiphenyl ether
- BDE 47
2,2′,4,4′-Tetrabromodiphenyl ether
- BDE 66
2,3′,4,4′-Tetrabromodiphenyl ether
- BDE 85
2,2′,3,4,4′-Pentabromodiphenyl ether
- BDE 99
2,2′,4,4′,5-Pentabromodiphenyl ether
- BDE 100
2,2′,4,4′,6-Pentabromodiphenyl ether
- BDE 153
2,2′m4,4′,5,5′-hexabromodiphenyl ether
- BDE 154
2,2′,4,4′,5,6′-Hexabromodiphenyl ether
- BDE 183
2,2′,3,4,4′,5′,6-Heptabromodiphenyl ether
- BDE 209
2,2′,3,3′,4,4′,5,5′,6,6′-Decabromodiphenyl ether
- BB 153
2,2′,4,4′,5,5′-Hexabromobiphenyl
Footnotes
Publisher's Disclaimer: Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention, the International Trachoma Initiative, the National Oceanic and Atmospheric Administration, or the National Institute of Standards and Technology. Commercial equipment, instruments, or materials are identified to specify adequately the experimental procedure. Such identification does not imply recommendation or endorsement by the above agencies.
1 Polychlorinated biphenyl (PCB) and polybrominated diphenyl ether (PBDE) congener nomenclature follows the IUPAC designation (IUPAC, 2017).
Declaration of competing interest
The authors have no conflicts of interest to disclose.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.scitotenv.2019.134041.
References
- Agency for Toxic Substances and Disease Registry (ATSDR), 2002. Toxicological profile for DDT, DDE, and DDD. Available online. https://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=81&tid=20, Accessed date: 9 January 2017. [PubMed]
- Agency for Toxic Substances and Disease Registry (ATSDR), 2016. Per- and polyfluoroalkyl substances and your health. Available online. https://www.atsdr.cdc.gov/pfc/index.html, Accessed date: 6 March 2017.
- Balmer BC, Schwacke LH, Wells RS, George RC, Hoguet J, Kucklick JR, Lane SM, Martinez A, McLellan WA, Rosel PE, Rowles TK, Sparks K, Speakman T, Zolman ES, Pabst DA, 2011. Relationship between persistant organic pollutants (POPs) and ranging patterns in common bottlenose dolphins (Tursiops truncates) from coastal Georgia, USA. Science Tot Env 10.1016/j.scitotenv.2011.01.052. [DOI] [PubMed]
- Bossart GD, 2010. Marine mammals as sentinel species for oceans and human health. Vet Pathology online 10.1177/0300985810388525 Available online. http://vet.sagepub.com/content/early/2010/12/11/0300985810388525, Accessed date: 17 May 2019. [DOI] [PubMed] [Google Scholar]
- Boucher O, Muckle G, Jacobson JL, Carter RC, Kaplan-Estrin M, Ayotte P, Dewailly E, Jacobson SW, 2014. Domain-specific effects of prenatal exposure to PCBs, mercury, and lead on infant cognition: results from the Environmental Contaminants and Child Development Study in Nunavik. Environ. Health Perspect 122, 310–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buha A, 2011. Mirex. Toxipedia.org Available online. http://www.toxipedia.org/display/toxipedia/Mirex, Accessed date: 12 January 2018.
- Buttke D, 2011. Toxicology, environmental health, and the “one health” concept. Journal of Medical Toxicology 7, 329 10.1007/s13181-011-0172-45(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, 2016. National Health and Nutrition Examination Survey (NHANES) MEC Laboratory Procedures Manual. National Center for Health Statistics, U.S; Available online. https://stacks.cdc.gov/view/cdc/44831 (Accessed 8 March 2018). [Google Scholar]
- Centers for Disease Control and Prevention (CDC), 2003. Laboratory Procedure Manual. Available at. https://www.cdc.gov/nchs/data/nhanes/nhanes_05_06/pbcd_d_met_lead_cadmium.pdf, Accessed date: 10 March 2017.
- Centers for Disease Control and Prevention (CDC), 2018. Fourth Report on Human Exposure to Environmental Chemicals, Updated Tables March. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Atlanta, GA, Atlanta, GA Available at: https://www.cdc.gov/exposurereport/. [Google Scholar]
- Dewailly E, Pereg D, Knap A, Rouja P, Galvin J, 2008. Exposure and effects of seafood-borne contaminants in maritime populations Chapter 10 In: Walsh PJ, Smith SL, Fleming LE, Solo-Garbriel HM, Gerwick WH (Eds.), Oceans and Human Health. Academic Press, New York, pp. 181–191. [Google Scholar]
- DeWit CA, 2002. An overview of brominated flame retardants in the environment. Chemosphere 46, 583–624. [DOI] [PubMed] [Google Scholar]
- Fair PA, Mitchum G, Hulsey TC, Adams J, Zolman E, McFee W, Wirth E, Bossart GD, 2007. Polybrominated diphenyl ethers (PBDEs) in blubber of free-ranging bottlenose dolphins (Tursiops truncatus) from two Southeast Atlantic estuarine areas. Arch Environ Comtam Toxicol 53, 483–494. [DOI] [PubMed] [Google Scholar]
- Fair PA, Houde M, Hulsey TC, Bossrt GD, Adams J, Balthis L, Muir DCG, 2012. Assessment of perflourinated compounds (PFCs) in plasma of bottlenose dolphins from two southeast US estuarine areas: relationship with age, sex, and geographic locations. Mar. Pollut. Bull 64, 66–74. [DOI] [PubMed] [Google Scholar]
- Fair PA, White ND, Wolf B, Arnott SA, Kannan K, Karthikraj R, Vena J, 2018. Persistent organic pollutants in fish frim Charleston Harbor and tributaries, South Carolina, United States: a risk assessment. J. Env. Res 1677, 598–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederiksen M, Vorkamp K, Thomsen M, Knudsen LE, 2009. Human internal and external exposure to PBDEDs – a review of levels and sources. ScienceDirect 212, 109–134. [DOI] [PubMed] [Google Scholar]
- Fromme H, Tittlemier SA, Volkel W, Wilhelm M, Twardella D, 2009. Perfluorinated compounds – exposure assessment for the general population in western countries. Int. J. Hyg. Environ. Health 212, 239–270. [DOI] [PubMed] [Google Scholar]
- Giesy J, Kannan K, 2001. Global distribution of perfluorooctane sulfonate in wildlife. Environ. Sci. Technol 35, 1339–1342. [DOI] [PubMed] [Google Scholar]
- Hites RA, 2004. Polybrominated diphenyl ethers in the environment and in people: a meta-analysis of concentrations. Environ Sci Technol 38, 945–956. [DOI] [PubMed] [Google Scholar]
- Hoguet J, Keller J, Reiner J, Kucklick J, Bryan C, Moors A, Pugh R, Becker PR, 2013. Spatial and temporal trends of persistent organic pollutants and mercury in beluga whales (Delphinapterus leucas) from Alaska. Sci. Total Environ 449, 285–294. [DOI] [PubMed] [Google Scholar]
- Houde M, Balmer B, Brandsma S, Wells RS, Rowles T, Solomon KR, Muir DC, 2006. Perfluoroalkyl compounds in relation to life-history and reproductive parameters from Sarasota Bay, Florida USA. Environ. Toxicol. Chem 25, 2405–2412. [DOI] [PubMed] [Google Scholar]
- Huckins JN, Stalling D, Petty JD, Johnson BT, 1982. Fate of kepone and mirex in the aquatic environment. J. Agricultural and Food Chemistry 30 10.1021/jf00114a004. [DOI] [Google Scholar]
- International Union of Pure and Applied Chemistry (IUPAC), 2017. Chemical nomenclature and structure representation. Available online. https://iupac.org/who-we-are/divisions/division-details/?body_code=800, Accessed date: 19 May 2017.
- Ishikawa Y, Falandysz J, Noma Y, Sakai S, 2005. Chlorobiphenyl constituents of Aroclor 1268, Chlorofen, Clophen T 64, KC-600, and KC-1000 technical formulations. Journal of Environmental Science and Health, Part A: Toxic/Hazardous Substances and Environmental Engineering 40 (12), 2171–2187. 10.1080/10934520500234668. [DOI] [PubMed] [Google Scholar]
- Jones R, Edenfield E, Anderson S, Zhang Y, Sjödin A, 2012. Semi-automated extraction and cleanup method for measuring persistent organic pollutants in human serum. Organohalogen Compd. 74, 97–98. [Google Scholar]
- Kannan K, Maruya KA, Tanabe S, 1997. Distribution and characterization of polychlorinated biphenyl congeners in soil and sediments from a superfund site contaminated with Aroclor 1268. Environmental Science & Technology 31 (5), 1483–1488. [Google Scholar]
- Kato K, Basden BJ, Needham LL, Calafat AM, 2011a. Improved selectivity for the analysis of maternal serum and cord serum for polyfluoroalkyl chemicals. J. Chromatogr. A 1218 (15), 2133–2137. [DOI] [PubMed] [Google Scholar]
- Kato K, Wong L-Y, Jia LT, Kuklenyik Z, Calafat AM, 2011b. Trends in exposure to polyfluroalkyl chemicals in the U.S. population: 1999–2008. Environ Sci Technol 45, 8037–8045. [DOI] [PubMed] [Google Scholar]
- Kucklick J, Schwacke L, Wells R, Hohn A, Guichard A, Yordy J, Hansen L, Zolman E, Wilson R, Litz J, Nowacek D, Rowles T, Pugh R, Balmer B, Sinclair C, Rosel P, 2011. Bottlenose dolphins as indicators of persistent organic pollutants in the western North Atlantic Ocean and Northern Gulf of Mexico. Environ Sci Technol 45, 4270–4277. [DOI] [PubMed] [Google Scholar]
- Kuklenyik Z, Needham LL, Calafat AM, 2005. Measurement of 18 perfluorinated organic acids and amides in human serum using on-line solid-phase extraction. Anal. Chem 77, 6085–6091. [DOI] [PubMed] [Google Scholar]
- Litz J, Garrison LP, Fieber LA, Martinez A, Contillo JP, Kucklick JR, 2007. Fine-scale spatial variation of persistent organic pollutants in bottle nose dolphins (Tursiops truncatus) in Biscayne Bay, Florida. Environ. Sci. Technol 41, 7222–7228. [DOI] [PubMed] [Google Scholar]
- Maruya KA, 1997. PCB contamination at the LCP Chemicals Superfund site, Brunswick, Georgia Proceedings of the 1997 Georgia Water Resources Conference, March 20–22, 1997. University of Georgia, Athens, Georgia: Available online. http://www.gwri.gatech.edu/sites/default.files/files/docs/1997.Maruyak-97.pdf, Accessed date: 6 January 2014. [Google Scholar]
- Maruya KA, Lee RF, 1998. Aroclor 1268 and toxaphene in fish from a Southeastern U.S. estuary. Environ Sci Technol 32, 1069–1075. [Google Scholar]
- Mexfish.com, d. Available online. http://www.mexfish.com/fish/fish.htm, Accessed date: 9 December 2015.
- Muir DCG, Backus S, Derocher AE, Dietz R, Evans TJ, Gabrielsen GW, Nagy J, Norstrom RJ, Sonne C, Stirling I, Taylor MK, Letcher RJ, 2006. Brominated flame retardants in polar bears (Ursus maritimus) from Alaska, the Canadian Arctic, East Greenland, and Svalbard. Environ Sci Technol 40, 449–455. [DOI] [PubMed] [Google Scholar]
- Myers GJ, Davidson PW, Cox C, Shamlaye CF, Palumbo D, Cernichiari El, Sloane-Reeves J, Wilding GE, Kost J, Huang L-S, Clarkson TW, 2003. Prenatal methyl-mercury exposure from ocean fish consumption in the Seychelles child development study. Lancet 361, 1686–1692. [DOI] [PubMed] [Google Scholar]
- National Pesticide Information Center, 1999. DDT. Available online. http://npic.orst.edu/factsheets/ddtgen.pdf, Accessed date: 12 January 2018.
- National Pesticide Information Center, Chlordane. Available online: http://npic.orst.edu/factsheets/chlordanegen.pdf (Accessed 12 January 2018).
- NIOSH, d. Adult blood lead epidemiology and surveillance (ABLES). Available online. https://www.cdc.gov/niosh/topics/ables/description.htm, Accessed date: 18 January 2018.
- Patterson DG Jr., Alexander LR, Turner WE, Isaacs SG, Needham LL, 1991. The development and application of a high resolution mass spectrometry method for measuring polychlorinated dibenzo-p-dioxins and dibenzofurans in serum Chapter 9 In: Clement RE, Sui KM, Hill HH Jr. (Eds.), Instrumentation for Trace Organic Monitoring. Lewis Publishers, Boca Raton. [Google Scholar]
- Rabinowitz P, Scotch M, Conti L, 2009. Human and animal sentinels for shared health risks. Vet. Ital 45 (1), 23–24. [PMC free article] [PubMed] [Google Scholar]
- Reif JS, 2011. Animal sentinels for environmental and public health. Public Health Rep. 126 (Suppl. 1), 50–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reif JS, Schaefer AM, Bossart GD, 2015. Atlantic bottlenose dolphins (Tursiops truncatus) as a sentinel for exposure to mercury in humans: closing the loop. Vet Sci 2, 407–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwacke LH, Zolman ES, Balmer BC, DeGuise S, George RC, Hoguet J, Hohn AA, Kucklick JR, Lamb S, Levin M, Litz JA, McFee WE, Place NJ, Townsend FI, Wells RS, Rowles TK, 2012. Anaemia, hypothyroidism and immune supporession associated with polychlorinated biphenyl exposure in bottlenose dolphins (Tursiops truncatus). Proc. R. Soc. B 279, 48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwope DM, Nienow CK, Mills WJ. 2005. University of Chicago http://www.sge.com/uploads/c4/81/c481d209d7a0ffa7986f351c9c7ed954/mills_dioxin_2005_final.pdf (Accessed 6 January 2014).
- Sjödin A, Patterson DG, Bergman A, 2003. A review on human exposure to brominated flame retardants—particularly polybrominated diphenyl ethers. Environ. Int 29, 829–839. [DOI] [PubMed] [Google Scholar]
- Sjödin A, Jones RS, Lapeza CR, Focant J-F, McGahee III EE, Patterson DG Jr.. 2004. Semi-automated high-throughput extraction and cleanup method for the measurement of polybrominated diphenyl ethers, polybrominated biphenyls and polychlorinated biphenyls in human serum. Anal. Chem 76:1921–1927. [DOI] [PubMed] [Google Scholar]
- Speardiver, d. Available online. http://www.speardiver.com/fish/724-fish-name-translation-english-spanish.html, Accessed date: 9 December 2015.
- Steenland K, Fletcher T, Savitz DA, 2010. Epidemiologic evidence on the health effects of perfluorooctanoic acid (PFOA). Environ. Health Perspect 118, 1100–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang-Péronard JL, Heitmann BL, Andersen HR, Steuerwald U, Grandjean P, Weihe P, Jensen TK, 2014. Association between prenatal polychlorinated biphenyl exposure and obesity development at ages 5 and 7 y: a prospective cohort study of 656 children from the Faroe Islands. Am. J. Clin. Nutr 99, 5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turyk M, Anderson H, Knobeloch L, Imm P, Persky V, 2009. Organochlorine exposure and incidence of diabetes in a cohort of Great Lakes fish consumption. Environ. Health Perspect 117 (7), 1076–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turyk ME, Bhavsar SP, Bowerman W, Boysen E, Clark M, Diamond M, Mergler D, Pantazopoulos P, Schantz S, Carpenter DO, 2012. Risks and benefits of consumption of Great Lakes fish. Environ Health Persepct 120, 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency (EPA), 1997. Test Methods for Evaluating Solid Waste, Method 3545 U.S. EPA SW-846, Update III Fed Regist 62(114) U.S. GPO, Washington, DC, p. 32451. [Google Scholar]
- U.S. Environmental Protection Agency (EPA), 2007. EPA Method 8290A (SW-846): Polychlorinated Dibenzodioxins (PCDDs) and Polychlorinated Dibenzofurans (PCDFs) by High-resolution Gas Chromatography/high Resolution Mass Spectrometry (HRGC/HRMS). Available online. https://www.epa.gov/hw-sw846/sw-846-test-method-8290a-polychlorinated-dibenzodioxins-pcdds-and-polychlorinated, Accessed date: 16 May 2018.
- U.S. Environmental Protection Agency (EPA), 2007a. EPA Method 8082A (SW-846): Polychlorinated Biphenyls (PCBs) by Gas Chromatography. Available online. https://www.epa.gov/homeland-security-research/epa-method-8082a-sw-846-polychlorinated-biphenyls-pcbs-gas-chromatography, Accessed date: 16 May 2018.
- U.S. Environmental Protection Agency (EPA), 2014. SW-846 Test Method 6020B: Inductively Coupled Plasma-mass Spectrometry. Available online. https://www.epa.gov/hw-sw846/sw-846-test-method-6020b-inductively-coupled-plasma-mass-spec-trometry, Accessed date: 30 September 2016.
- U.S. Environmental Protection Agency (EPA), 2016. Assessing and Managing Chemicals under TSCA. Per- and Polyfluoroalkyl Substances (PFASs) Under TSCA. Available online. https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/andpolyfluoroalkyl-substances-pfass-under-tsca, Accessed date: 6 March 2016. [Google Scholar]
- U.S. Environmental Protection Agency (EPA), 2017. Technical Fact Sheet—Polybrominated Diphenyl Ethers (PBDEs). Available online. https://www.epa.gov/sites/production/files/2014-03/documents/ffrrofactsheet_contaminant_perchlorate_january2014_final_0.pdf, Accessed date: 13 March 2018.
- Vestergren R, Cousins IT, 2009. Tracking the pathways of human exposure to perfluorocarboxylates. Environ Sci Technol 43, 5565–5575. [DOI] [PubMed] [Google Scholar]
- Wirth EF, Pennington PL, Cooksey C, Schwacke L, Balthis L, Hyland J, Fulton MH, 2014. Distribution and sources of PCS (Aroclor 1268) in the Sapelo Island National Estuarine Research Reserve. Environ. Monit. Assess 186, 8717 10.1007/s10661-014-4039-4. [DOI] [PubMed] [Google Scholar]
- Yordy J, Jucklick JR, Wells RS, Balmer BC, Schwacke L, Rowles T, 2010. Partitioning of persistant organic pollutants (POPs) between blubber and blood of wild bottlenose dolphins: implications for biomonitoring and health. Environ. Sci. Technol 44 (12), 4789–4795. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


