Figure 4: Evaluating the influence of WT Fbg αC (233–435) on thrombin-catalyzed cleavage of the FXIII A2 and FXIII A2B2 activation peptide.
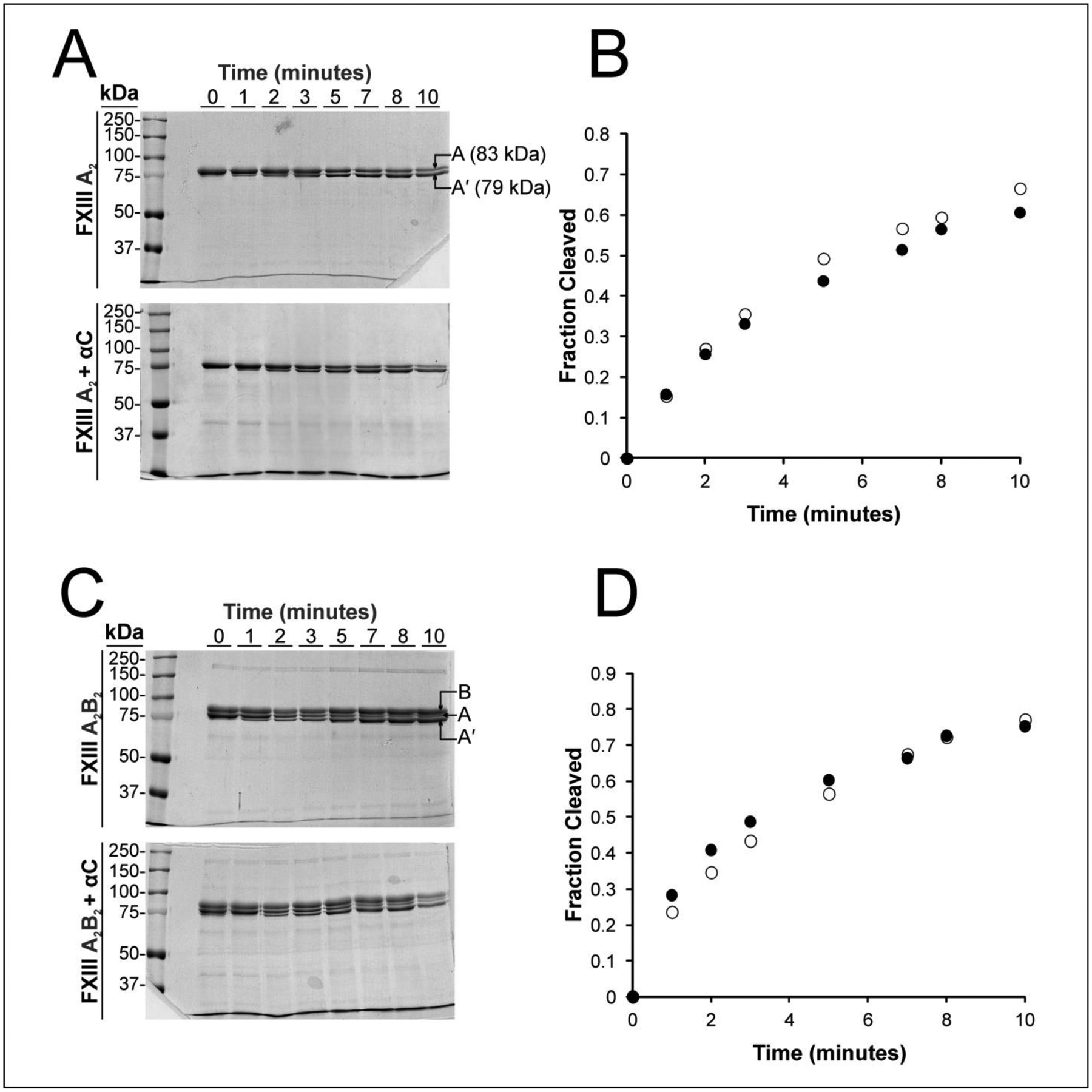
Cleavage reactions contained 5 μM αC (233–425), 1μM FXIIIA (from FXIII A2 or FXIII A2B2), and 4mM CaCl2 in Tris-acetate buffer. Incubations were maintained at 37°C and quenched at defined time points using the thrombin inhibitor PPACK. Samples were run under reducing conditions on 8% SDS-PAGE gels and stained with Coomassie Blue. A) Gels showing reactions with FXIII A2 (top) versus FXIII A2 + αC (233–425) bottom. Following cleavage of the FXIII activation peptide segment at the R37-G38 peptide bond, the MW for the A subunit (83 kDa) decreases to A’ (79 kDa). In the lower figure, the dark band found at the bottom of the gel corresponds to αC (233–425). B) The gels were dried and the fractions of FXIII A-chains cleaved over time were calculated using Image J. The filled circles in the plot correspond to free FXIII A2 and the open circle to FXIII A2 in the presence of αC (233–425). The αC region does not seem to promote thrombin cleavage of FXIII A2. C) Gels showing reactions with FXIII A2B2 (top) versus FXIII A2B2 + αC (233–425) bottom. Following cleavage of the FXIII activation peptide, the MW for the A subunit (83 kDa) decreases to A’ (79 kDa). D) The filled circles in the plot correspond to free FXIII A2B2 and the open circle to FXIII A2B2 in the presence of αC (233–425). The αC region does not seem to promote thrombin cleavage of FXIII A2B2. For both FXIII forms, representative data from a set of independent triplicates are displayed.
