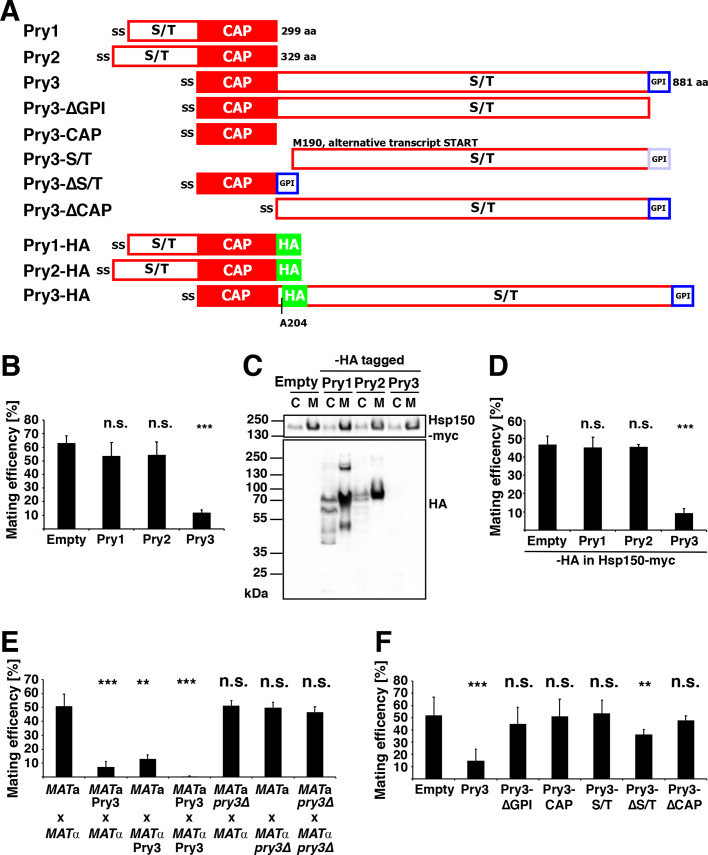
Fig. 1.
The CAP domain of Pry3 is required for mating inhibition. (A) Domain structure of the yeast CAP family members and schematic illustration of the truncated versions of Pry3 tested for their ability to confer mating inhibition. The three yeast CAP family members Pry1, Pry2 and Pry3 are composed of a conserved CAP domain (red box) and a serine/threonine-rich domain (S/T) of varying length. All three proteins enter the secretory pathway due to the presence of an N-terminal signal sequence (ss). Pry3 is unusual in that it contains the serine/threonine-rich region downstream of the CAP domain and the protein is GPI-anchored (blue box). The GPI-anchor of Pry3-S/T is highlighted in light blue, since the sequence should not be recognized as GPI-anchor due to the absence of the signal sequence. Deletion constructs of Pry3 tested for functionality in a mating assay, Pry3-ΔGPI, Pry3-CAP, Pry3-S/T, Pry3-ΔS/T and Pry3-ΔCAP are depicted. The positions of the HA tags at the C-termini of Pry1 and 2 and the internal tag in Pry3, placed after alanine 204, are represented by green boxes. (B) Among the yeast CAP proteins, only Pry3 overexpression inhibits mating. Cells transformed with an empty plasmid or overexpressing either Pry1, Pry2 or Pry3 were mated to wild-type cells of the opposite mating type for 5 h at 30°C. Cells were then plated on solid media and mating efficiency was quantified and plotted. (C) Pry1 and Pry2 are secreted glycoproteins, Pry3 is not detectable by western blotting. Protein extracts from total cells (C) and culture media (M) expressing HA-tagged Pry1, Pry2 and Pry3 were analyzed by western blotting. Detection of the secreted Hsp150-myc serves as secretion control. (D) HA-tagged Pry3 is functional in mating inhibition. Mating efficiency of cells transformed with an empty plasmid or overexpressing either HA-tagged Pry1, Pry2 or Pry3. (E) Mating inhibition by Pry3 is additive. Cells of the indicated mating types either overexpressing (Pry3) or lacking Pry3 (pry3Δ) were tested for mating efficiency. (F) The GPI-anchored CAP domain of Pry3 is sufficient for mating inhibition. Mating efficiency of cells containing an empty plasmid, overexpressing wild-type Pry3, or the indicated deletion variants of Pry3. Values for mating efficiency shown in panels B, D, E and F represent means±s.d. of four independent determinations. Asterisks denote statistical significance (Welch t-test; **P-value <0.01; ***P-value <0.001; n.s., not significant).