Figure 2.
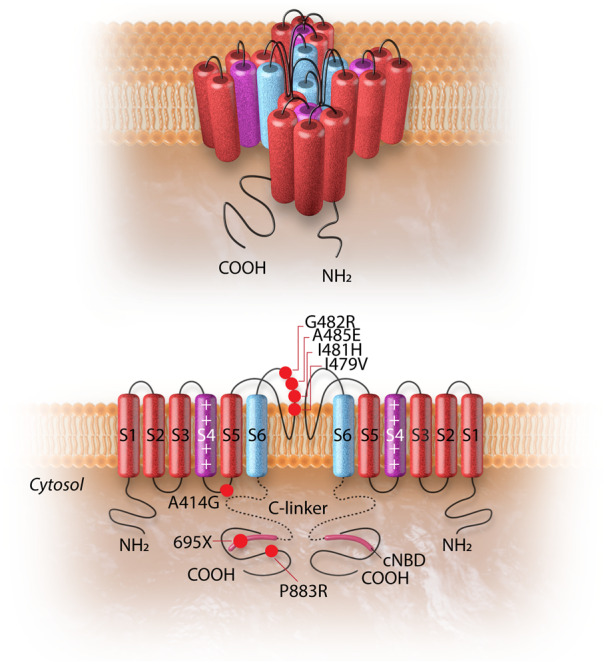
The HCN4 channel and the topology of the HCN4 mutations associated with left ventricular non-compaction cardiomyopathy. The upper panel shows the 3D conformational structure of the HCN4 channel. The lower panel shows the liner structure of the HCN4 channel (two out of four subunits are shown). Each alpha-subunit is composed of six transmembrane helixes (S1–S6), a pore-forming loop, and intracellular N- and C-termini. The positively charged S4 helix (shown in purple) is the voltage sensor of the channel. The four S6 segments (shown in blue) of four-channel monomers together form the ion-conducting passage of the HCN4 channel. The C-terminus comprises of the C-linker (dotted line) and the cyclic nucleotide-binding domain (cNBD), which is connected to the channel core and mediates cyclic AMP-dependent changes in HCN channel gating. cAMP binding causes a conformation change that leads to the assembly of an active tetramer and channel opening. Red dots on the alpha-subunit shown on left indicate the sites of mutation associated with left ventricular non-compaction. The dot with a black dash indicates a truncation resulting from an insertion mutation leading to premature truncation of the protein at amino acid 695.
