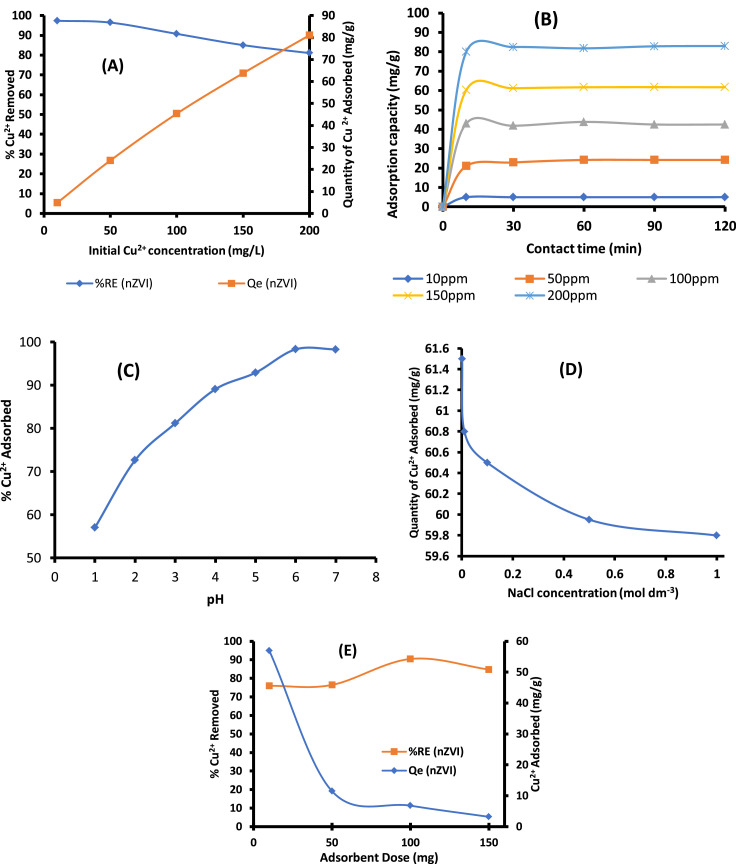
Fig. 6.
(A): Effect of initial Concentration on adsorption of ED-ions Cu(II) onto CS-nZVI. Experimental conditions:Vol of Cu2+solution = 50 mL; mg/LAdsorbent dose = 100 mg;pH = 6, contact time = 60 min and temperature = 25± 2 °C. (B): Effect of contact time on adsorption of ED-Cu(II) onto CS-nZVI. Experimental conditions:Cu2+Concentration=10 – 200 mg L-1; vol of Cu2+solution = 50 mL;Adsorbent dose= 100 mg; pH = 6, and temperature = 25± 2 °C. (C): Effect of pH on adsorption of ED-Cu(II)ions onto ions CS-nZVI. Experimental conditions: Vol of Cu2+solution = 50 mL; Adsorbent dose = 100 mg;pH = 6, contact time = 60 min and temperature = 25± 2 °C, Stirring speed = 200 rpm. (D): Effect of ionic strength on adsorption of ED-Cu(II) ions onto CS-nZVI. Experimental conditions:Cu2+Concentration=10 – 200 mg/L; Vol of Cu2+solution = 50 mL;Adsorbent dose= 100 mg; pH = 6, and temperature = 25± 2 °C, Stirring speed =200 rpm. (E): Effect of adsorbent dose on adsorption of ED-Cu(II) ion on CS-nZVI. Experimental conditions: Cu2+ Concentration= 150 mg/L; Vol of Cu2+solution = 50 mL; pH = 6, Stirring speed = 200 rpm, Contact time = 60 min, and Temperature = 25± 2 °C.