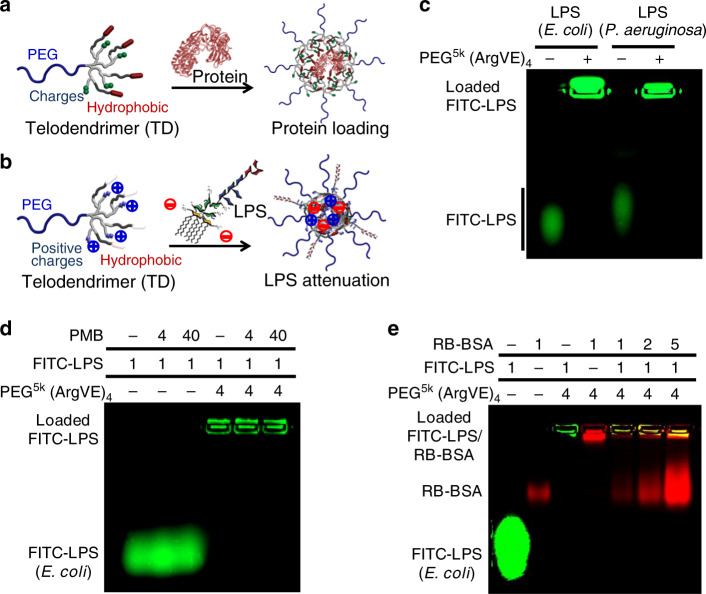
Fig. 1. Protein and LPS binding in telodendrimer nanoparticles.
Schematic illustration of a protein and b LPS captured by telodendrimer nanoparticles via the combination of charge and hydrophobic interactions. c–e Agarose gel electrophoresis profiles reveal the complex formation of the FITC-labled LPS with telodendrimer PEG5k(ArgVE)4, as indicated by the lost mobility in migration (repeated independently a minimum of twice). c LPS originated from both E. coli and P. aeruginosa can be captured efficiently by the telodendrimer PEG5k(ArgVE)4. d PMB form less-stable complex with LPS in electrophoresis, which was also unable to dissociate LPS– PEG5k(ArgVE)4 nanocomplex with 40-fold excess in mass ratio. e The stability of LPS–PEG5k(ArgVE)4 nanocomplex was also observed to be stable in the presence of serum protein (RB-BSA) at different mass ratios.