Figure 5.
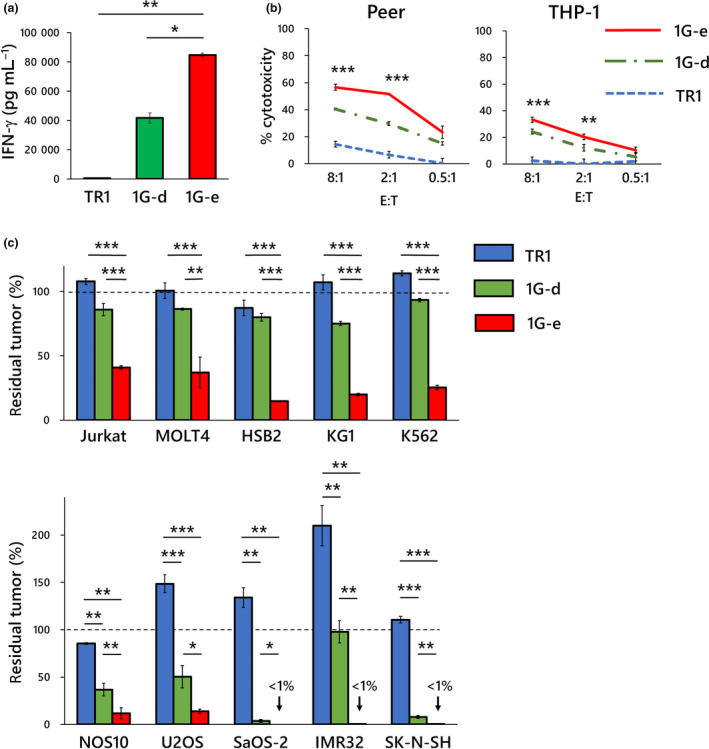
Hinge region of NKp44 is involved in ligand binding and recognition in NKp44‐based CAR. The impact of the hinge domain of NKp44‐based CAR on CAR function was examined. (a) NKp44‐based CAR‐T cells with alternative hinge domain (1G‐d; green square), in which NKp44‐original hinge was replaced by CD8α hinge, produced significantly lower levels of IFN‐γ than NKp44‐based CAR‐T cells with the original hinge (1G‐e; red square) after 24‐h exposure to target cells (KG‐1) as measured by cytometric beads array. In both CAR‐T cells used for the experiments, surface expression levels of CAR on gene‐modified T cells were similar (Figure 1b). (b) NKp44‐based CAR‐T cells with an alternative hinge domain (1G‐d; green chain line) showed inferior 4‐h cytotoxicity as compared with NKp44‐based CAR‐T cells with the original hinge (1G‐e; red solid line). As shown by the asterisks, statistical comparisons between 1G‐d and 1G‐e CAR‐T cells in both cell lines at E:T ratio of 8:1 and 2:1 were significantly different. (c) NKp44‐based CAR‐T cells with the alternative hinge domain (1G‐d; green square) showed significantly lower long‐term anti‐tumor effects in 7 days of co‐culture assay, as compared to NKp44‐based CAR‐T cells with the original hinge (1G‐e; red square) against various leukaemia (at an E:T ratio of 0.5:1; upper graph) and solid tumor cell lines (at an E:T ratio of 4:1). The y‐axis shows percentage of residual tumors (as compared with tumor cells without effector cells). These observations suggest an important role of the original NKp44 hinge domain in CAR function including antigen recognition and signalling. Data are means ± SD of three technical replicates. Experiments were independently repeated at least twice, and representative data are shown. *P < 0.05, **P < 0.01, ***P < 0.001.
