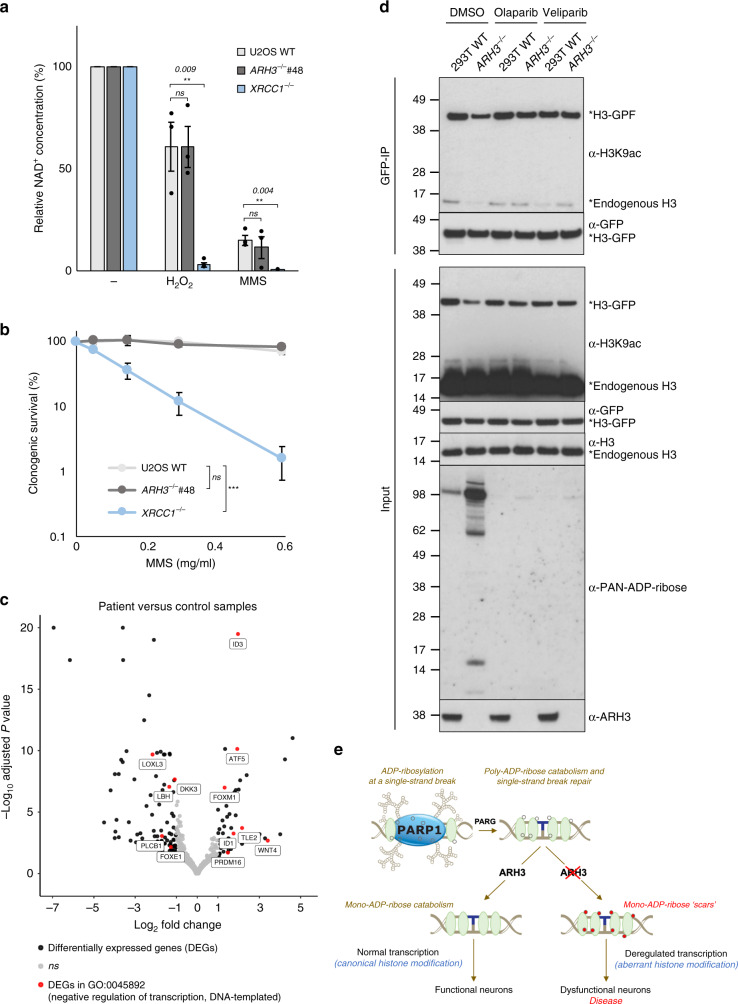
Fig. 6. Deregulated transcription in ARH3-defective cells.
a NAD+ depletion in wild-type, ARH3−/− and XRCC1−/− U2OS cells following DNA damage. Wild-type, ARH3−/− and XRCC1−/− U2OS cells were harvested 45 min after mock-treatment or treatment with either H2O2 or MMS and NAD+ concentrations determined by a chromogenic assay. Data are the mean ± SEM of three independent experiments. Statistical analysis (one-tail t-test) is indicated (**P < 0.01; ns, not significant). b Clonogenic survival of wild-type, ARH3−/− and XRCC1−/− U2OS cells following treatment with the indicated concentrations of MMS. Data are the mean ± SEM of three independent experiments. Statistical analysis (two-way analysis of variance) is shown (ns, not significant; ***P < 0.001; 9.644E-10). c Volcano plot of patient vs control transcription profiles showing differentially expressed genes (DEGs; adjusted P-value < 0.05, Log2 fold change > 1; see Methods). DEGs shown in red are placed in the significantly enriched Gene ontology (GO) category GO:0045892 (negative regulation of transcription, DNA − templated). d ADP-ribose chromatin scars result in reduced levels of histone H3K9 acetylation. Wild-type and ARH3−/− 293 T cells were transfected with plasmid encoding H3-GFP and incubated with DMSO vehicle or with PARP inhibitors (Olaparib or Veliparib), as indicated. 24 h later, GFP-tagged nucleosomes were immunoprecipitated and examined by western blotting for levels of H3K9 acetylation, H3-GFP, endogenous H3, ADP-ribose, and ARH3. Input samples are also shown (bottom panels). e A model for the formation and impact of persistent mono-ADP-ribose scars in neurological disease. PARP1 is activated at endogenous SSBs, modifying itself and nearby histones (green ovals) with mono-ADP-ribose (single open circles) and/or poly-ADP-ribose (chains of open circles). Following the completion of SSBR, the de novo mono-ADP-ribose moieties and/or those resulting from PARG activity remain. The mono-ADP-ribose is removed by ARH3 in normal cells, but persists in ARH3 patient cells, forming a chromatin ‘scar’ and providing a ‘memory’ of previously repaired SSBs. The persistent mono-ADP-ribose in ARH3-defective chromatin impedes local histone acetylation and likely other nearby histone modifications, resulting in a perturbed histone code, deregulated transcription, and cellular dysfunction.