Abstract
We tested the social complexity hypothesis which posits that animals living in complex social environments should use complex communication systems. We focused on two components of vocal complexity: diversity (number of categories of calls) and flexibility (degree of gradation between categories of calls). We compared the acoustic structure of vocal signals in groups of macaques belonging to four species with varying levels of uncertainty (i.e. complexity) in social tolerance (the higher the degree of tolerance, the higher the degree of uncertainty): two intolerant species, Japanese and rhesus macaques, and two tolerant species, Tonkean and crested macaques. We recorded the vocalizations emitted by adult females in affiliative, agonistic and neutral contexts. We analysed several acoustic variables: call duration, entropy, time and frequency energy quantiles. The results showed that tolerant macaques displayed higher levels of vocal diversity and flexibility than intolerant macaques in situations with a greater number of options and consequences, i.e. in agonistic and affiliative contexts. We found no significant differences between tolerant and intolerant macaques in the neutral context where individuals are not directly involved in social interaction. This shows that species experiencing more uncertain social interactions displayed greater vocal diversity and flexibility, which supports the social complexity hypothesis.
Keywords: acoustics, social system, social style, cluster analysis, comparison, primates
1. Introduction
When looking for the determinants of social evolution in animals, two main types of factors can be distinguished: external pressures coming from the environment and internal constraints arising from the structure of the phenotype. Understanding how adaptation to environmental factors shapes social behaviour has attracted a great deal of research, and is in fact a main objective of the field of behavioural ecology [1,2]. In comparison, the role of structural constraints in biology has long been a controversial issue [3,4], and much less effort has been devoted to studying how they channel social organizations [5]. Although the definition of structural constraints itself has been problematic for some time, they can be actually defined as processes that limit the response of phenotypic traits to the selective action of ecological factors [6,7]. These constraints arise from the existence of functional relationships that link phenotypic traits or from passive interconnections that have occurred over the course of evolutionary history, and keep them in an entrenched state [5,8,9]. According to the social complexity hypothesis for communicative complexity, there is a functional relationship between patterns of communication and patterns of social organization: animals living in complex social environments should use complex communication systems because a complex social life increases the need to discriminate individuals, express a wide range of emotional states, and convey a broad variety of messages related to different goals and contexts [10–12]. Although the social complexity hypothesis applies to communicative signals, in general, most of the current evidence comes from the study of vocal communication [10]. The correlations found between the amount of information or the size of vocal repertoire on one side, and the size of social groups [13–15] or the number of categories of individuals on the other side [11,16], are in line with this hypothesis. However, there are problems with the definition and measurement of both social and vocal complexity.
There is no consensus on measures of the complexity of social systems [10,17–19]. The number of individuals in a social unit, as well as their number of categories or interactions, have long been used as indicators of complexity [10,11,14,16,17,20]. More recently, authors have focused on the number of social relationships or associations between group members [18,21]. Numbering the components of social systems may provide a good proxy for assessing their diversity, but diversity is only part of complexity, it does not encompass all aspects of complexity [22], which limits the evaluation of the social complexity hypothesis.
A similar problem hinders the measurement of the complexity of vocal communication [23]. Authors generally assume that the greater the number of call types, the higher the level of vocal complexity [14,15,24]. In these studies, what is considered is the diversity of communication signals rather than the complexity of the entire vocal system. Moreover, there is no agreement on how to identify the types of calls, and therefore the size of a species' communicative repertoire [23]. The task is especially tricky when repertoires are graded, that is, when there is gradual transition from one acoustic structure into another [23], as reported in species such as primates [25,26]. Some have proposed abandoning the idea of counting the number of calls to quantify vocal complexity, and instead using the degree of gradation of repertoires [23,27], i.e. flexibility in the acoustic structure of vocal signals. Because diversity and flexibility represent two different components of complexity, however, it seems that the best solution is to take both into account when characterising vocal complexity [22].
Uncertain outcomes appear to be the most important characteristic of complex systems [28,29]. Shannon's information theory [30] provides a way to quantify diversity and flexibility in terms of uncertainty [22]. This theory refers to what can be treated as a quantity of information which is here synonymous with a lack of a priori knowledge about the outcome of events, and therefore their unpredictability. More types of calls or more graded calls offer a greater number of options and, ultimately, the greater the number of options, the greater the uncertainty. The social complexity hypothesis can, therefore, be tested by comparing the diversity and flexibility of communication in species with varying levels of uncertainty in their social relationships. These species must be close enough to allow for homologous comparison from the point of view of both social relations and communication signals. In this respect, the genus Macaca offers a model that meets these requirements. Macaque species exhibit wide variations in their degree of social tolerance, which can be related to different levels of uncertainty in the outcome of their agonistic interactions [31,32]. In the most intolerant species, social conflicts generally have clear consequences: in Japanese macaques (Macaca fuscata) and rhesus macaques (Macaca mulatta), for instance, the recipient of aggression flees or submits in nine out of 10 cases among unrelated females [33]. By contrast, in more tolerant species the recipient of the aggression frequently protests or counter-attacks: in Tonkean macaques (Macaca tonkeana) and crested macaques (Macaca nigra), 68.0 and 45.4% of conflicts among unrelated females, respectively, remain undecided, with no clear winners and losers [33].
The need for complex communication signals is not necessarily the same in all social contexts [10]. In the agonistic context, animals need information to cope with the many potential outcomes of uncertain situations such as open contests between two or more individuals, which affects competition for resources and exposes individuals to risk of injury. In the affiliative context, a wealth of communication signals can also help individuals to achieve the best solution from a variety of behavioural options and maintain their social relationships [24,34]. Significant interspecies differences in communication systems are to be expected in situations of competition and cooperation. On the contrary, no significant interspecies differences should occur in neutral circumstances—i.e. when individuals are not directly involved in a social interaction—that do not require the expression of a wide range of intentions.
The interspecific variations reported in the agonistic patterns of macaques covary with other components of their social style such as hierarchical steepness, degree of nepotism, reconciliation rates, or range of facial displays; for example, dominance and kinship relations have stronger influence on individual behaviours in intolerant macaques compared with tolerant macaques, and the latter reconcile more often and have a greater number of facial displays than the former [31,35,36]. Despite such variations, macaque species share the same basic patterns of organization. All are semi-terrestrial primates living in multimale-multifemale groups; males disperse, and females remain in their natal group where they constitute matrilines, i.e. subgroups of relatives linked by maternal descent [35]. While no association has been found so far between the contrasting social styles of macaque species and the ecological conditions in which they have evolved, it appears that social styles consistently vary with phylogeny: closely related species are more similar than those that are distant [5,36,37].
In this study, we compared the vocal signals of two tolerant species (Tonkean and crested macaques, M. tonkeana and M. nigra) and two intolerant species (Japanese and rhesus macaques, M. fuscata and M. mulatta), based on three main variables (acoustic distance, diversity and flexibility) in three different social contexts (agonistic, affiliative and neutral). Like the other species of macaque, they use a graded repertoire of vocalizations [38–41]. They are mainly frugivorous and their primary habitat is forest, with the exception of rhesus macaques which occur in a variety of habitats, from forests to arid lands or regions of human settlement [37]. Both Tonkean and crested macaques live on different parts of the island of Sulawesi, Indonesia, they belong to the oldest macaque lineage [42]. Japanese and rhesus macaques live in Japan and mainland southern Asia, respectively, and both belong to a more recent lineage [42,43]. The two lineages separated about 5 Ma [44,45]. In comparison, the divergence between Tonkean and crested macaques on one side, and Japanese and rhesus macaques on the other side, is much more recent. It is estimated to have occurred almost 1 Ma at the latest [45,46]. Because of these phylogenetic distances, it can be expected that the vocal signals used by individuals will differ more between these two pairs of species than within each pair. However, such differences should apply indiscriminately to the various vocal variables and social contexts, contrary to the social complexity hypothesis which specifies that contrasts between species should depend on the variables and contexts.
We tested the predictions of three different hypotheses: (i) null hypothesis: we should find no significant difference in the calls of tolerant and intolerant species regardless of variables and contexts; (ii) phylogenetic hypothesis: greater similarity should occur in more closely related species, for any variable, and regardless of the social context, so we should find more differences between Tonkean and crested macaques on the one hand, and Japanese and rhesus macaques on the other, than within each of these species pairs across variables and contexts; and (iii) social complexity hypothesis: greater uncertainty in the social interactions of tolerant species compared to intolerant species should be associated with greater vocal diversity and flexibility in the former species than in the latter, while no significant differences should be found regarding the acoustic distances of calls. In addition, differences in diversity and flexibility should vary across social contexts: they should be apparent in the agonistic and affiliative contexts, and absent in the neutral context.
2. Methods
(a). Subjects and living conditions
We made behavioural observations and acoustic recordings in 29 adult females from two groups of Japanese macaques, 16 adult females from two groups of rhesus macaques, 13 adult females from four groups of Tonkean macaques and 51 adult females from two groups of crested macaques. We focused on adult females because they are the most represented age-and-sex category in macaque social groups, and also the most active contributors in vocal communication [47]. Japanese, rhesus and Tonkean macaque females were captive born and at least 5 years old. Crested macaques were studied in their natural habitat, and the age of the subjects was assessed according to their reproductive history since 2006 (Macaca Nigra Project, www.macaca-nigra.org), their body size, the shape of their nipples, and the presence of old physical injuries. The composition of groups is given in the electronic supplementary material, table S1.
The groups of Japanese macaques (Ft, Fw) were housed in two enclosures of 960 and 4600 m2, respectively, at the Primate Research Institute in Inuyama, Japan [48]. The groups of rhesus macaques (Ma, Mb) were housed in two 210 m2 enclosures at the Biomedical Primate Research Center in Rijswijk, The Netherlands [49]. One group of Tonkean macaques (Tb) was housed at the Orangerie Zoo in Strasbourg, France, in a 120 m2 enclosure, and the other three groups (Tc, Td, Te) were housed at the Parco Faunistico di Piano dell'Abatino Rescue Centre in Rieti, Italy, in 500 m2 enclosures [49]. Enclosures were wooded or furnished with perches, ropes and shelters. Animals were fed commercial monkeys diet pellets, supplemented with fresh fruits and vegetables, and water was available ad libitum. The groups of crested macaques (Npb, Nr1) lived in the Tangkoko Nature Reserve, North Sulawesi, Indonesia [34]. They were not provisioned and inhabit lowland tropical rainforest [50].
The study complied with the legal requirements and guidelines of the Italian, French, Dutch, Indonesian and Japanese governments, and followed the ASAB/ABS guidelines for the treatment of animals in behavioural research. In what follows we will refer for convenience to the Tonkean and crested macaque species as the Tonkean/crested pair, and the Japanese and rhesus macaque species as the Japanese/rhesus pair.
(b). Data collection
We carried out observations outdoors to ensure the quality of the recordings. Data were collected by A.L. in Japanese macaques [48], N.R. in rhesus macaques, A.D.M., A.S. and N.R. in Tonkean macaques [49] and J.M. in crested macaques [34] (electronic supplementary material, table S1). We observed subjects in a predefined random order using focal sampling. Sample duration was 10 min in Japanese and Tonkean macaques from groups Tc, Td and Te, 15 min in rhesus macaques and Tonkean macaques from group Tb, and 30 min in crested macaques. This resulted in 6.1 ± 0.16 h of focal sampling per female in Japanese macaques, 12.7 ± 0.7 h in rhesus macaques, 13.6 ± 3.2 h in Tonkean macaques and 7.8 ± 0.4 in crested macaques.
In Japanese macaques, we recorded vocalizations with a TCD-D100 Sony (Tokyo, Japan) DAT recorder (WAV format, sampling frequency: 44 100 Hz, resolution: 16 bits), and an ECM672 Sony directional microphone. In rhesus and Tonkean macaques, we used a Marantz (Eindhoven, The Netherlands) PMD661 recorder (WAV format, sampling frequency: 44 100 Hz, resolution: 16 bits), and a Sennheiser (Wedermark, Germany) K6 and ME66 directional microphone. In crested macaques, we used partly a high-resolution camera Panasonic (Osaka, Japan) HDC-SD700 linked to a Sennheiser (Wedermark, Germany) K6 and ME66 directional microphone, and partly a Marantz (Eindhoven, The Netherlands) PMD661 (WAV format, sampling frequency: 32 000 Hz, resolution: 16 bits). We collected observational data about the context of call emission with a lavalier microphone connected to the recorder in Japanese, rhesus and Tonkean macaques (at805f, audio-technica, Leeds, UK versus TCM160, Meditec, Singapore). In the crested macaques, the observer filmed the focal individual while a field assistant recorded contextual data using a handheld computer; we extracted the audio tracks from the video recordings using the software FFmpeg (v. 3.4.1).
We distinguished three social contexts: agonistic, affiliative and neutral. Contexts were defined according to the behaviours that could occur in the 3 s before and after the emission of a call or a sequence of calls. A sequence was itself defined as a series of calls separated by a maximum of 3 s. Note that behaviour patterns could fluctuate before and after the emission of the calls, but the context did not change. Behavioural units were based on published repertoires for macaques [51–53]. The agonistic context included aggression (supplantation, lunge, chase, slap, grab, bite, facial threat display) and response to aggression (aggression, avoidance, flight, crouch, submissive facial displays). The affiliative context included affiliative behaviours (approach, sitting in contact, social grooming, social play, grasp, embrace, mount, affiliative facial display). In the neutral context, the caller was not involved in a social interaction.
(c). Acoustic analysis
We had records for 1368 calls in Japanese macaques, 1026 calls in rhesus macaques, 1210 calls in Tonkean macaques and 1234 calls in crested macaques. The first author (N.R.) drew spectrograms using the software Raven Pro v. 1.4’ (Cornell Laboratory of Ornithology, Center for Conservation Acoustics, Ithaca, NY, USA) with a 256 fast Fourier transform length and a Hanning window. With the same software, she measured the following variables: duration: duration from the beginning to the end of a call, in seconds; Q2 ratio: ratio between duration that divides a call into two intervals of equal energy and duration in percentage; Q1 frequency: value of the frequency that divides a call into two intervals containing 25% and 75% of the energy, in Herz; Q2 frequency: value of the frequency that divides a call into two intervals of equal energy, in Herz; Q3 frequency: value of the frequency that divides a call into two intervals containing 75% and 25% of the energy, in Herz; Wiener's aggregate entropy: degree of disorder (i.e. noisiness) of the call, which uses the total energy in a frequency bin over the entire call; and Wiener's average entropy: mean of the mean entropies of the different time slices of a call. Our objective was to compare the four species on tonal and atonal calls, so we did not take into account the variables associated with fundamental frequencies because they are absent in atonal calls.
We selected recordings according to their quality. We randomly selected no more than three calls per sequence. A sequence was defined as a series of calls separated by a maximum of 3 s. Based on the total number of calls, females with a sample size of less than five calls were excluded from the analysis. We also excluded some specific types of calls for which we could collect only a few recordings or none in each species: alarm calls, œstrus calls and twits and cackles. Our samples resulted in 434 calls in 24 Japanese macaques (agonistic context: total number of calls, 79 and mean number of calls per female ± s.d., 3.30 ± 377; affiliative context: 94 and 3.92 ± 4.16; neutral context: 255 and 10.6 ± 5.48), 639 calls in 16 rhesus macaques (agonistic: 118 and 7.38 ± 6.75; affiliative: 59 and 3.69 ± 3.22; neutral: 461 and 28.8 ± 16.0), 700 calls in 13 Tonkean macaques (270 and 20.8 ± 26.3, 226 and 17.4 ± 14.3, 202 and 15.5 ± 8.42), and 696 calls in 19 crested macaques (201 and 10.6 ± 6.61, 297 and 15.6 ± 11.8, 191 and 10.1 ± 7.40).
(d). Statistical analysis
Statistical analyses were run in R (version 3.6.1) [54]. In a first analysis, we tested the differences in acoustic variables between species. In a second analysis, we assessed vocal diversity and compared it across species; we first performed a principal component analysis (PCA), then a cluster analysis using an algorithm adapted to the graded repertoire. In a third analysis, we quantified the degree of gradation of the repertoire based on assignment probabilities using a second cluster analysis.
(i). Acoustic distances
To test the differences between species in their acoustic variables, we performed discriminant function analyses using the function lda of the package MASS [55]. Because a discriminant function analysis can be affected by the unit in which predictor variables are measured, we scaled the acoustic variables prior to analysis. As collinearity can bias the results of a linear discriminant analysis [56], we removed acoustic variables so that each Pearson pairwise correlation between acoustic variables was less than 0.7; a simulation study showed that this is the value above which collinearity begins to bias model estimates, and is consequently the most commonly used threshold [57]. We, therefore, included the following variables in the discriminant function analysis: duration, Q2 ratio, Q2 frequency, average entropy. We used the function PermuteLDA from the package multiDimBio [58] to assess interspecific differences in acoustic variables that we name acoustic distances, which allowed us to statistically determine whether the species were at different locations in the multivariate space [59]. The function PermuteLDA calculated the multivariate distances between the sets of calls of each species in each context, and determined whether they differed significantly using Monte Carlo randomization.
PCA: as individuals were described by multifactorial characteristics, we used PCA to reduce the dimensionality of the dataset and provide more stable clustering, which means that clustering outputs are less sensitive to outliers [60]. In addition, the PCA approach eliminates correlations between factors that can influence clustering. Prior to PCA, and per context for all species, we scaled the seven acoustic variables to obtain a standard deviation of one, and a mean of zero, using the R base function scale [54]. The PCAs per context were then performed using the PCA function of the FactoMineR package [61]. We weighted each female according to her number of calls by applying the argument row.w of the PCA function to balance the contributions of the different females to the creation of the space. Eventually, we selected the number of dimensions that explained near 95% of the variance of the data.
(ii). Vocal diversity
It is possible to measure vocal diversity by the number of call types in the repertoire of a species [12]. We ourselves measured it using the number of main categories of calls (i.e. groups of calls with similar acoustic characteristics) as follows. There is more uncertainty in communication when individuals can emit more calls, i.e. when the number of groups of calls is large. We determined the diversity in groups of calls by quantifying the number of clusters that structured the dataset. The greater the number of clusters, the greater the vocal diversity. To calculate the optimal number of clusters, we chose to apply Gaussian mixture models (GMMs) based on a clustering approach [62–64]. GMMs assume that the clusters come from a finite mixture of probability distributions, which allows each group to be described with a different volume, shape, and orientation. The distribution parameters must be computed, which has been done by an expectation maximization algorithm. The best model was then selected based on the Bayesian information criterion (BIC) score. The BIC scoring of a GMM was performed using the function Mclust of the package Mclust [65]. We have considered only the optimal number of clusters defined by the best model. As we wanted to compare these optimums statistically between each of the species, we used a bootstrap procedure. We ran 100 bootstraps where 80% of the data was sampled per bootstrap.
(iii). Vocal flexibility
We can measure signal uncertainty as the degree of gradation between call types [22]. We named vocal flexibility the degree of gradation between calls: the higher vocal flexibility is, the greater is the potential for information transmission [12]. We used the probability for a single call to belong to the different clusters to measure the degree of gradation between clusters. Accordingly, we used the soft assignment from a fuzzy clustering algorithm over GMM because we aimed at avoiding shape, volume or orientations difference between groups that can affect the likelihood of membership to each cluster. We applied the function fanny from the package cluster [66]. We set the argument membership exponent at 1.2 because it was the highest value—giving a higher degree of fuzziness [67]—that did not lead to a convergence issue. Each call was assigned a probability of belonging to each cluster (n probabilities per call for n clusters). Therefore, if a call had a probability of one to belong to cluster A, and of zero to belong to any other clusters, this call was considered as typical of cluster A. On the contrary, if a call had more evenly distributed probabilities, it was considered as an intermediate call between at least two different clusters. The higher the number of intermediates, the higher the degree of gradation between clusters. Hence, to quantify this degree, we could use the Shannon's entropy formula [30]: the higher the entropy, the more even the distribution across clusters. We calculated the entropy of each call. Entropy value was then transformed into a relative entropy value, i.e. the entropy divided by the logarithm of the number of clusters [68,69]. We then calculated the mean of these relative entropy values. This computation was performed for a number of clusters varying from two to six (optimal number of clusters range).
(iv). Statistical comparisons
We compared the optimal number of clusters between species with a generalized linear model (GLM) using a Poisson family. We compared the entropy value (i.e. degree of gradation between clusters) using linear models (LM). We compared the full models (i.e. with species as predictor factor) to the null models (i.e. without species) by applying likelihood ratio tests (LRT) using the function lrtest of the package lmtest [70]. This allowed us to assess whether the species factor had a significant effect. When species had a significant effect, we performed post hoc tests to make pairwise comparisons using the function emmeans of the package emmeans [71].
3. Results
(a). Acoustic distance
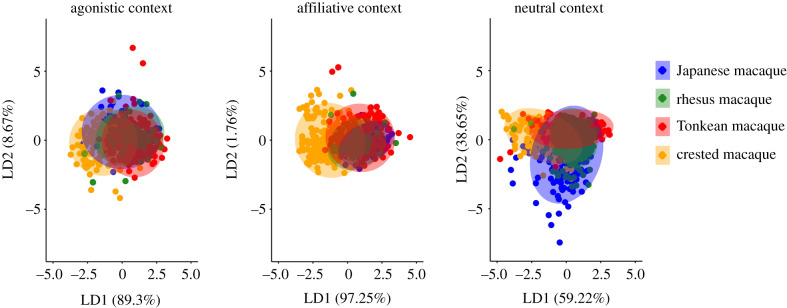
In the agonistic context, pairwise comparisons in the multivariate acoustic distances yielded significant differences between species, except between Japanese and Tonkean macaques; the distances between rhesus and Tonkean macaques remained limited relative to other distances between species (figure 1; electronic supplementary material, table S2). In the affiliative context, comparisons also yielded significant differences, except between Japanese and rhesus macaques; the distances between Tonkean macaques and either Japanese or rhesus macaques were limited (figure 1; electronic supplementary material, table S2). In the neutral context, all pairwise comparisons produced significant differences, but distances between Japanese, rhesus and Tonkean macaques were limited; crested macaques were farther from the other species in the three contexts (figure 1; electronic supplementary material, table S2). As an outcome, no grouping appeared between the Tonkean and crested macaques on one side, and Japanese and rhesus macaques on the other side.
Figure 1.
Comparisons of acoustic distances between species for calls emitted in the agonistic, affiliative and neutral contexts: linear discriminant analysis biplot with the four groups centroids of species on the first two linear discriminants (LD1 and LD2). The ellipses correspond to the 95% confidence interval. (Online version in colour.)
(b). Vocal diversity
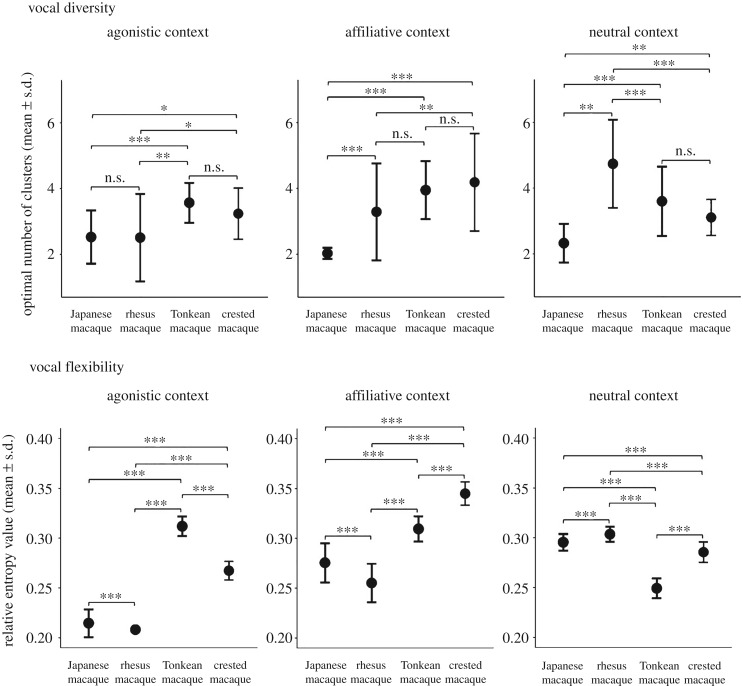
In the agonistic context, the mean optimal number of clusters differed significantly between species (LRT χ2 = 28.1, p < 0.001), meaning that they differed in their number of groups of calls. Post hoc tests revealed that the Tonkean/crested pair had a significantly greater number of clusters than the Japanese/rhesus pair; no significant differences were found between the two members of each pair (Tonkean/crested macaques pair; Japanese/rhesus pair) (figure 2; electronic supplementary material, table S3). In the affiliative context, the mean optimal number of clusters differed significantly between species (LRT χ2 = 90.4, p < 0.001). Post hoc tests showed that the Japanese macaques had a significantly smaller number of clusters than the other species; rhesus macaques had a lower number of clusters than the Tonkean/crested pair although the difference was significant with the crested macaques and not with the Tonkean macaques; Tonkean and crested macaques did not differ in their numbers of clusters (figure 2; electronic supplementary material, table S3). In the neutral context, the mean optimal number of clusters differed significantly between species (LRT χ2 = 88.3, p < 0.001). Post hoc tests revealed that rhesus macaques had a significantly greater number of clusters than the other species; Tonkean macaques had a similar number of clusters compared to crested macaques; Japanese macaques had a significantly smaller number of clusters than the other species (figure 2; electronic supplementary material, table S3).
Figure 2.
Comparisons of vocal diversity and flexibility between species for calls emitted in the agonistic, affiliative and neutral contexts: optimal numbers of clusters and entropy values (***p < 0.001, **p < 0.01, *p < 0.05, n.s., non-significant).
We used the truncation of the mean optimal number (n) of clusters for each species and context to illustrate the optimal grouping of call types usually recognized in macaque species (see the electronic supplementary material, tables S4 and S2, three-dimensional cluster graphs). Although call types such as screams, barks and coos were common to the four species, other types of calls were specific to species: girneys and growls in Japanese and rhesus macaques, and soft grunts, hard grunts and chuckles in Tonkean and crested macaques (electronic supplementary material, table S4).
(c). Vocal flexibility
In the agonistic context, the mean entropy value was significantly different between species (LRT χ2 = 1092, p < 0.001), meaning that they varied in the degree of gradation between call types. Post hoc tests showed that the strongest differences opposed the Japanese/rhesus pair to the Tonkean/crested pair, with the latter displaying higher entropies than the former. Additionally, Tonkean macaques had a higher entropy than crested macaques, and Japanese macaques had a higher entropy than rhesus macaques (figure 2; electronic supplementary material, table S3). In the affiliative context, the entropy value was significantly different between species (LRT χ2 = 679, p < 0.001). Post hoc tests revealed that the strongest differences opposed the Japanese/rhesus pair to the Tonkean/crested pair, with the Tonkean/crested pair displaying a higher entropy than the Japanese/rhesus pair; crested macaques had a higher entropy than Tonkean macaques, and Japanese macaques had a higher entropy than rhesus macaques (figure 2; electronic supplementary material, table S3). In the neutral context, the entropy value was significantly different between species (LRT χ2 = 737, p < 0.001). Post hoc tests revealed no clear pattern contrasting the Japanese/rhesus to the Tonkean/crested pairs; rhesus macaques had a higher entropy compared to the other species; Japanese macaques had a higher entropy compared to Tonkean and crested macaques, and crested macaques had a higher entropy than Tonkean macaques (figure 2; electronic supplementary material, table S3).
4. Discussion
Based on the comparison of the acoustic variables characterizing both tonal and atonal calls, we found that the vocalizations of the four species of macaques studied differed by several respects. Although call types such as screams, barks and coos were common to all of them, other types of calls were specific to species, consistently with the results of previous studies: girneys and growls in Japanese and rhesus macaques, and soft grunts, hard grunts and chuckles in Tonkean and crested macaques [38,39,72–74]. The analysis of the acoustic distances between the sets of calls recorded in each species for each context confirmed that each macaque species has its own acoustic repertoire [41]. In particular, we did not find any significant contrasts in acoustic distances that would allow us to arrange the sets of calls of Japanese macaques and rhesus on one side, and Tonkean and crested macaques on the other side.
We addressed vocal diversity by identifying the optimal number of groups of calls in each species. This showed that the Japanese/rhesus pair differed from the Tonkean/crested pair in the agonistic context; the latter had one additional group of calls compared to the former. It should be emphasized that a group of calls does not represent a single type of call, but generally includes several types. In other words, this means that the diversity of call types was more extensive in Tonkean and crested macaques compared to Japanese and rhesus macaques in the context of aggression. We found a similar pattern in the affiliative context, although the difference between rhesus and Tonkean macaques was not statistically significant. On the other hand, we did not find similar contrasts between the two pairs of species in the neutral context. We also examined vocal flexibility by analysing the degree of gradation between groups of calls. We found the same type of demarcation between the Japanese/rhesus and the Tonkean/crested pairs in the agonistic and the affiliative contexts. As for vocal diversity, no difference appeared in the neutral context between both pairs of species.
Based on the interspecies contrasts evidenced in the acoustic structure of calls, we can reject the null hypothesis that there should be no difference between the Tonkean/crested and Japanese/rhesus pairs. The phylogenetic hypothesis posits that closely related species should show generalized similarity in calls for any acoustic variable and social context. However, this fails to explain why the two pairs of species differed in the number of groups of calls and the degree of gradation between calls, but not in their acoustic distances, nor why the contrasts were consistent in the agonistic and affiliative contexts, but not in the neutral context. By contrast, the social complexity hypothesis is able to account for these various results. This hypothesis predicts that only complexity variables—vocal diversity measured by the number of groups of calls and vocal flexibility measured by the degree of gradation—should differ between the Tonkean/crested and Japanese/rhesus pairs, and exclusively in the agonistic and affiliative contexts. Indeed, we found that species differences in the neutral context did not follow any pattern related to variations in the degree of social uncertainty between pairs of species. As callers do not receive specific responses from their group mates in the neutral context, the number of possible outcomes remain limited, and it is understandable that vocal complexity was not influenced by the species-specific style of social interactions.
The social interactions of tolerant macaque species are characterized by a higher degree of freedom than those of more intolerant macaques, as they are less constrained by kinship and dominance relations [75]. Functionally, a greater diversity of vocal signals and a marked gradation between them can provide richer and more nuanced meanings, as moving gradually from one display to another would allow the signals to express a broad motivational spectrum [76]. In other words, such signals have the potential to contain a large amount of information and convey a wide range of emotions and intentions. This would contribute to the developed negotiation skills of tolerant macaques, enabling them to engage in highly sophisticated affiliative interactions, manage undecided open contests, and achieve high rates of conflict resolution [34,77–81].
It should be stressed that our results are by nature correlational. The causal direction of the social complexity hypothesis for communicative complexity is still debated [12]. While complex social situations may require complex communicative abilities, complex communicative abilities may also foster the emergence of complex social interactions. Because the two processes are not mutually exclusive, a positive feedback loop may occur between them at the evolutionary level. In addition, it is generally assumed that the social complexity hypothesis applies to entire social systems. Our results reveal that the hypothesis can hold for some social situations and not for others. In particular, we did not find consistent differences between tolerant and intolerant macaques in the neutral context, where most of the recorded calls were coos and growls. As mentioned above, it is logical that no link between social and communicative complexity has emerged in a context where callers were not involved in social interactions.
We have studied the calls of three species of macaque in captive settings, and in the wild for the fourth, but we found no contrast between groups that could be attributed to the recording conditions. Furthermore, while Japanese, Tonkean and crested macaques are mainly forest-dwelling species, rhesus macaques can live in quite diverse habitats. Again, our analyses did not reveal systematic contrasts between rhesus macaques and the other three species. It is known that the physical structure of the habitat can affect the frequency or amplitude of auditory signals for example [25,82], but we have relied on variables related to vocal diversity and flexibility, for which no influence of ecological conditions is assumed to date [10]. Future research should confirm the contrasts in vocal diversity and flexibility found between tolerant and intolerant macaques by extending the analyses to a larger number of groups and species. The additional study of the combinations of calls in vocal sequences and the responses of receivers will also be necessary to test the social complexity hypothesis in a comprehensive way.
Supplementary Material
Supplementary Material
Acknowledgements
We are grateful to the managers and keepers of the Parco Faunistico di Piano dell'Abatino (Rieti), the Orangerie Zoo (Strasbourg), the Biomedical Primate Research Center (Rijswijk) and the Primate Research Institute (Inuyama) for their valuable assistance. We thank S. Louazon and V. Biquand for their technical help, and F. Husson for his statistical advice. Data collection in crested macaques was supported by the Indonesian State Ministry of Research and Technology (RISTEK), the Directorate General of Forest Protection and Nature Conservation (PHKA), the Department for the Conservation of Natural Resources (BKSDA, North Sulawesi), the staff of the Macaca Nigra Project and the Agricultural University of Bogor.
Ethics
Research was observational. It complied with the legal requirements and guidelines of the Italian, French, Dutch and Japanese governments, and followed the ASAB/ABS guidelines for the treatment of animals in behavioural research.
Data accessibility
The dataset is available at the Dryad Digital Repository https://doi.org/10.5061/dryad.905qfttgw [83].
Authors' contributions
Experimental design: N.R., B.T., A.L.; animal management: A.D.M., A.S., J.A.M.L., E.H.M.S.; data collection: N.R., A.D.M., A.S., A.L., J.M.; data analysis: N.R, A.D.M, A.S, J-C.L.; manuscript writing: N.R., B.T.; critical comments and additions to the manuscript: all authors; funding: R.C.
Competing interests
We declare we have no competing interests.
Funding
The work was supported by the Fondazione Ethoikos (Radicondoli), the Fondation des Treilles (Tourtour), and the Department of Ecology, Physiology and Ethology (Strasbourg). Data collection in rhesus macaques was supported by an Eole Scholarship granted by the French-Dutch Network. Data collection in Japanese macaques was supported by the Japan Society for the Promotion of Science.
References
- 1.Danchin E, Giraldeau LA, Cézilly F. 2008. Behavioural ecology. Oxford, UK: Oxford University Press. [Google Scholar]
- 2.Davies NB, Krebs JR, Stuart AW. 2012. An introduction to behavioural ecology. Oxford, UK: Wiley-Blackwell. [Google Scholar]
- 3.Gould SJ, Lewontin RC. 1979. The spandrels of San Marco and the Panglossian paradigm: a critique of the adaptationist programme. Proc. R. Soc. B 205, 581–598. [DOI] [PubMed] [Google Scholar]
- 4.Maynard Smith J, Burian R, Kauffman S, Alberch P, Campbell J, Goodwin B, Lande R, Raup D, Wolpert L. 1985. Developmental constraints and evolution. Q. Rev. Biol. 60, 265–287. ( 10.1086/414425) [DOI] [Google Scholar]
- 5.Thierry B. 2013. Identifying constraints in the evolution of primate societies. Phil. Trans. R. Soc. B 368, 20120342 ( 10.1098/rstb.2012.0342) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antonovics J, van Tienderen PH. 1991. Ontoecogenophyloconstraints? The chaos of constraint terminology. Trends Ecol. Evol. 6, 166–168. ( 10.1016/0169-5347(91)90059-7) [DOI] [PubMed] [Google Scholar]
- 7.Schlichting CD, Pigliucci M.. 1998. Phenotypic evolution. Sunderland, MA: Sinauer. [Google Scholar]
- 8.Brooks DR, McLennan DA.. 2013. The nature of diversity. Chicago, IL: University of Chicago Press. [Google Scholar]
- 9.Wimsatt WC, Schank JC. 2004. Generative entrenchement, modularity and evolvability: when genic selection meets the whole organism. In Modularity in development and evolution (eds Schlosser G, Wagner G), pp. 359–394. Chicago, IL: University of Chicago Press. [Google Scholar]
- 10.Freeberg TM, Dunbar RIM, Ord TJ. 2012. Social complexity as a proximate and ultimate factor in communicative complexity. Phil. Trans. R. Soc. B 367, 1785–1801. ( 10.1098/rstb.2011.0213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pollard KA, Blumstein DT. 2012. Evolving communicative complexity: insights from rodents and beyond. Phil. Trans. R. Soc. B 367, 1869–1878. ( 10.1098/rstb.2011.0221) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peckre LR, Kappeler PM, Fichtel C. 2019. Clarifying and expanding the social complexity hypothesis for communicative complexity. Behav. Ecol. Sociobiol. 73, 11 ( 10.1007/s00265-018-2605-4) [DOI] [Google Scholar]
- 13.Wilkinson GS, et al. 2019. Kinship, association and social complexity in bats. Behav. Ecol. Sociobiol. 73, 1–15. ( 10.1007/s00265-018-2608-1) [DOI] [Google Scholar]
- 14.Freeberg TM. 2006. Social complexity can drive vocal complexity: group size influences vocal information in Carolina chickadees. Psychol. Sci. 17, 557–561. ( 10.1111/j.1467-9280.2006.01743.x) [DOI] [PubMed] [Google Scholar]
- 15.McComb K, Semple S. 2005. Coevolution of vocal communication and sociality in primates. Biol. Lett. 1, 381–385. ( 10.1098/rsbl.2005.0366) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blumstein DT, Armitage KB. 1997. Does sociality drive the evolution of communicative complexity? A comparative test with ground-dwelling sciurid alarm calls. Am. Nat. 150, 179–200. ( 10.1086/286062) [DOI] [PubMed] [Google Scholar]
- 17.Bergman TJ, Beehner JC. 2015. Measuring social complexity. Anim. Behav. 103, 203–209. ( 10.1016/j.anbehav.2015.02.018) [DOI] [Google Scholar]
- 18.Fischer J, Farnworth MS, Hammerschmidt K, Sennhenn-Reulen H, Hammerschmidt K. 2017. Quantifying social complexity. Anim. Behav. 130, 57–66. ( 10.1016/j.anbehav.2017.06.003) [DOI] [Google Scholar]
- 19.Kappeler PM. 2019. Social complexity: patterns, processes, and evolution. Behav. Ecol. Sociobiol. 73, 5 ( 10.1007/s00265-018-2613-4) [DOI] [Google Scholar]
- 20.Lehmann J, Dunbar RIM. 2009. Network cohesion, group size and neocortex size in female-bonded old world primates. Proc. R. Soc. B 276, 4417–4422. ( 10.1098/rspb.2009.1409) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weiss MN, Franks DW, Croft DP, Whitehead H. 2019. Measuring the complexity of social associations using mixture models. Behav. Ecol. Sociobiol. 73, 8 ( 10.1007/s00265-018-2603-6) [DOI] [Google Scholar]
- 22.Rebout N, Lone JC, De Marco A, Cozzolino R, Lemasson A, Thierry B. Submitted. Measuring complexity in organisms and organizations. [DOI] [PMC free article] [PubMed]
- 23.Fischer J, Wadewitz P, Hammerschmidt K. 2017. Structural variability and communicative complexity in acoustic communication. Anim. Behav. 134, 229–237. ( 10.1016/j.anbehav.2016.06.012) [DOI] [Google Scholar]
- 24.Gustison ML, le Roux A, Bergman TJ. 2012. Derived vocalizations of geladas (Theropithecus gelada) and the evolution of vocal complexity in primates. Phil. Trans. R. Soc. B 367, 1847–1859. ( 10.1098/rstb.2011.0218) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hauser MD. 1996. The evolution of communication. Cambridge, MA: MIT Press. [Google Scholar]
- 26.Liebal K, Waller BM, Burrows AM, Slocombe KE (eds) 2014. Primate communication. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 27.Wadewitz P, Hammerschmidt K, Battaglia D, Witt A, Wolf F, Fischer J. 2015. Characterizing vocal repertoires: hard vs. soft classification approaches. PLos ONE 10, e0125785 ( 10.1371/journal.pone.0125785) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schuster P. 2016. How complexity originate: examples from history reveal additional roots to complexity. Complexity 21, 7–12. [Google Scholar]
- 29.McDaniel RRJ, Driebe DJ. 2005. Uncertainty and surprise in complex systems. Berlin, Germany: Springer. [Google Scholar]
- 30.Shannon CE. 1948. A mathematical theory of communication. Bell Syst. Tech. J. 27, 379–423. ( 10.1002/j.1538-7305.1948.tb01338.x) [DOI] [Google Scholar]
- 31.Dobson SD. 2012. Coevolution of facial expression and social tolerance in macaques. Am. J. Primatol. 74, 229–235. ( 10.1002/ajp.21991) [DOI] [PubMed] [Google Scholar]
- 32.Zannella A, Stanyon R, Palagi E. 2017. Yawning and social styles: different functions in tolerant and despotic macaques (Macaca tonkeana and Macaca fuscata). J. Comp. Psychol. 131, 179–188. ( 10.1037/com0000062) [DOI] [PubMed] [Google Scholar]
- 33.Thierry B, Aureli F, Nunn CL, Petit O, Abegg C, de Waal FBM. 2008. A comparative study of conflict resolution in macaques: insights into the nature of trait covariation. Anim. Behav. 75, 847–860. ( 10.1016/j.anbehav.2007.07.006) [DOI] [Google Scholar]
- 34.Micheletta J, Engelhardt A, Matthews L, Agil M, Waller BM. 2013. Multicomponent and multimodal lipsmacking in crested macaques (Macaca nigra). Am. J. Primatol. 75, 763–773. ( 10.1002/ajp.22105) [DOI] [PubMed] [Google Scholar]
- 35.Thierry B. 2007. Unity in diversity: lessons from macaque societies. Evol. Anthropol. 16, 224–238. ( 10.1002/evan.20147) [DOI] [Google Scholar]
- 36.Balasubramaniam KN, et al. 2017. The influence of phylogeny, social style, and sociodemographic factors on macaque social network structure. Am. J. Primatol. 80, 1–15. [DOI] [PubMed] [Google Scholar]
- 37.Ménard N. 2004. Do ecological factors explain variation in social organization? In Macaque societies (eds Thierry B, Singh M, Kaumanns W), pp. 157–185. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 38.Rowell TE, Hinde RA. 1962. Vocal communication by the rhesus monkey (Macaca mulatta). Proc. Zool. Soc. Lond. 138, 279–294. ( 10.1111/j.1469-7998.1962.tb05698.x) [DOI] [Google Scholar]
- 39.Green SM. 1975. Variation of vocal pattern with social situation in the Japanese monkey (Macaca fuscata): a field study. In Primate behavior (ed. Rosenblum LA.), pp. 1–102. New York, NY: Academic Press. [Google Scholar]
- 40.Masataka N, Thierry B. 1993. Vocal communication of Tonkean macaques in confined environments. Primates 34, 169–180. ( 10.1007/BF02381388) [DOI] [Google Scholar]
- 41.Gouzoules H, Gouzoules S. 2000. Agonistic screams differ among four species of macaques: the significance of motivation-structural rules. Anim. Behav. 59, 501–512. ( 10.1006/anbe.1999.1318) [DOI] [PubMed] [Google Scholar]
- 42.Fooden J. 1980. Classification and distribution of living macaques (Macaca Lacépède). In The macaques (ed. Lindburg DG.), pp. 1–9. New York, NY: Van Nostrand Rheinhold. [Google Scholar]
- 43.Delson E. 1980. Fossil macaques, phyletic relationships and a scenario of deployment. In The macaques (ed. Lindburg DG.), pp. 10–30. New York, NY: Van Nostrand Rheinhold. [Google Scholar]
- 44.Tosi AJ, Morales JC, Melnick DJ. 2003. Paternal, maternal, and biparental molecular markers provide unique windows onto the evolutionary history of macaque monkeys. Evolution 57, 1419–1435. ( 10.1111/j.0014-3820.2003.tb00349.x) [DOI] [PubMed] [Google Scholar]
- 45.Ziegler T, Abegg C, Meijaard E, Perwitasari-Farajallah D, Walter L, Hodges JK, Roos C. 2007. Molecular phylogeny and evolutionary history of Southeast Asian macaques forming the M. silenus group. Mol. Phylogenet. Evol. 42, 807–816. ( 10.1016/j.ympev.2006.11.015) [DOI] [PubMed] [Google Scholar]
- 46.Morales JC, Melnick DJ. 1998. Phylogenetic relationships of the macaques (Cercopithecidae: Macaca), as revealed by high resolution restriction site mapping of mitochondrial ribosomal genes. J. Hum. Evol. 34, 1–23. ( 10.1006/jhev.1997.0171) [DOI] [PubMed] [Google Scholar]
- 47.Lemasson A, Guilloux M, Barbu S, Lacroix A, Koda H. 2013. Age- and sex-dependent contact call usage in Japanese macaques. Primates 54, 283–291. ( 10.1007/s10329-013-0347-5) [DOI] [PubMed] [Google Scholar]
- 48.Arlet ME, Jubin R, Masataka N, Lemasson A. 2015. Grooming-at-a-distance by exchanging calls in non-human primates. Biol. Lett. 11, 20150711 ( 10.1098/rsbl.2015.0711) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De Marco A, Rebout N, Massiot E, Sanna A, Sterck EHM, Langermans JAM, Cozzolino R, Thierry B, Lemasson A. 2019. Differential patterns of vocal similarity in tolerant and intolerant macaques. Behaviour 156, 1–25. ( 10.1163/1568539X-00003562) [DOI] [Google Scholar]
- 50.Rosenbaum B, O'Brien TG, Kinnaird MF, Supriatna J. 1998. Population densities of Sulawesi crested black macaques (Macaca nigra) on Bacan and Sulawesi, Indonesia: effects of habitat disturbance and hunting. Am. J. Primatol. 44, 89–106. () [DOI] [PubMed] [Google Scholar]
- 51.Altmann SA. 1962. A field study of the sociobiology of rhesus monkeys (Macaca mulatta). Ann. N. Y. Acad. Sci. 102, 338–435. ( 10.1111/j.1749-6632.1962.tb13650.x) [DOI] [PubMed] [Google Scholar]
- 52.Fedigan LM. 1976. A study of roles in the Arashiyama West troop of Japanese monkeys (Macaca fuscata). Basel, Switzerland: Karger. [PubMed] [Google Scholar]
- 53.Thierry B, Bynum EL, Baker S, Kinnaird MF, Matsumura S, Muroyama Y, O'Brien TG, Petit O, Watanabe K. 2000. The social repertoire of Sulawesi macaques. Primate Res. 16, 203–226. ( 10.2354/psj.16.203) [DOI] [Google Scholar]
- 54.R Core Team. 2018. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 55.Venables WN, Ripley BD. 2002. Modern applied statistics with S. New York, NY: Springer. [Google Scholar]
- 56.Næs T, Mevik BH. 2001. Understanding the collinearity problem in regression and discriminant analysis. J. Chemom. 15, 413–426. ( 10.1002/cem.676) [DOI] [Google Scholar]
- 57.Dormann C, et al. 2012. Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography 36, 27–46. ( 10.1111/j.1600-0587.2012.07348.x) [DOI] [Google Scholar]
- 58.Scarpino SV, Gillette R, Crews D. 2013. MultiDimBio: an R package for the design, analysis, and visualization of systems biology experiments. See https://cran.r-project.org/web/packages/multiDimBio/index.html.
- 59.Collyer ML, Adams DC. 2012. Analysis of two-state multivariate phenotypic change in ecological studies. Ecology 88, 683–692. ( 10.1890/06-0727) [DOI] [PubMed] [Google Scholar]
- 60.Ben-Hur A, Guyon I. 2003. Detecting stable clusters using principal component analysis. In Functional genomics (eds Brownstein MJ, Khodursky AB), pp. 159–182. Berlin, Germany: Springer. [DOI] [PubMed] [Google Scholar]
- 61.Lê S, Josse J, Husson F. 2008. FactoMineR: an R package for multivariate analysis. J. Stat. Softw. 25, 1–18. [Google Scholar]
- 62.Everitt BS. 2014. Finite mixture distributions. See https://cran.r-project.org/web/packages/FactoMineR/index.html.
- 63.McNicholas PD. 2016. Model-based clustering. J. Classif. 33, 331–373. ( 10.1007/s00357-016-9211-9) [DOI] [Google Scholar]
- 64.Goeffrey M, Peel D. 2000. Finite mixture models. New York, NY: Wiley. [Google Scholar]
- 65.Scrucca L, Fop M, Murphy TB, Raftery AE, Riaz N, Wolden SL, Gelblum DY, Eric J. 2016. Mclust 5: clustering, classification and density estimation using Gaussian finite mixture models. R J. 8, 289–317. ( 10.32614/RJ-2016-021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maechler M, Rousseeuw P, Struyf A, Hubert M, Hornik K, Studer M, Roudier P. 2018. Cluster: cluster analysis basics and extensions. See https://cran.r-project.org/web/packages/cluster/index.html.
- 67.Kaufman L, Rousseeuw P. 1990. Finding groups in data. New York, NY: Wiley. [Google Scholar]
- 68.Peet RK. 1974. The measurement of species diversity. Annu. Rev. Ecol. Syst. 5, 285–307. ( 10.1146/annurev.es.05.110174.001441) [DOI] [Google Scholar]
- 69.Pielou EC. 1969. An introduction to mathematical ecology. New York, NY: Wiley. [Google Scholar]
- 70.Zeileis A, Hothorn T. 2002. Diagnostic checking in regression relationships. R News 2, 7–10. [Google Scholar]
- 71.Lenth R, Singmann H, Love J, Buerkner P, Herve M. 2018. Emmeans: estimated marginal means, aka least-squares means. See https://cran.r-project.org/web/packages/emmeans/index.html.
- 72.Hinde RA, Rowell TE. 1962. Communication by postures and facial expressions in the rhesus monkey (Macaca mulatta). Proc. Zool. Soc. Lond. 138, 1–21. ( 10.1111/j.1469-7998.1962.tb05684.x) [DOI] [Google Scholar]
- 73.Lindburg DG. 1971. The rhesus monkey in North India: an ecological and behavioural study. In Primate behavior (ed. Rosenblum LA.), pp. 1–106. Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- 74.Lewis SA. 1985. The vocal repertoire of the Celebes black ape (Macaca nigra). PhD thesis, University of Georgia, Athens, GA, USA. [Google Scholar]
- 75.Duboscq J, Neumann C, Agil M, Perwitasari-Farajallah D, Thierry B, Engelhardt A. 2017. Degrees of freedom in social bonds of crested macaque females. Anim. Behav. 123, 411–426. ( 10.1016/j.anbehav.2016.11.010) [DOI] [Google Scholar]
- 76.Morton ES. 1977. On the occurrence and significance of motivation-structural rules in some bird and mammal sounds. Am. Nat. 111, 855–869. ( 10.1086/283219) [DOI] [Google Scholar]
- 77.Petit O, Thierry B. 1994. Aggressive and peaceful interventions in conflicts in Tonkean macaques. Anim. Behav. 48, 1427–1436. ( 10.1006/anbe.1994.1378) [DOI] [Google Scholar]
- 78.De Marco A, Cozzolino R, Dessì-Fulgheri F, Thierry B. 2011. Collective arousal when reuniting after temporary separation in Tonkean macaques. Am. J. Phys. Anthrop. 146, 457–464. ( 10.1002/ajpa.21606) [DOI] [PubMed] [Google Scholar]
- 79.De Marco A, Sanna A, Cozzolino R, Thierry B. 2014. The function of greetings interactions in male Tonkean macaques. Am. J. Primatol. 76, 989–998. ( 10.1002/ajp.22288) [DOI] [PubMed] [Google Scholar]
- 80.Puga-Gonzalez I, Butovskaya M, Thierry B, Hemelrijk CK. 2014. Empathy versus parsimony in understanding post-conflict affiliation in monkeys: model and empirical data. PLoS ONE 9, e91262 ( 10.1371/journal.pone.0091262) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Duboscq J, Agil M, Engelhardt A, Thierry B. 2014. The function of postconflict interactions: new prospects from the study of a tolerant species of primate. Anim. Behav. 87, 107–120. ( 10.1016/j.anbehav.2013.10.018) [DOI] [Google Scholar]
- 82.Waser PM, Brown CH. 1986. Habitat acoustics and primate communication. Am. J. Primatol. 10, 135–154. ( 10.1002/ajp.1350100205) [DOI] [PubMed] [Google Scholar]
- 83.Rebout N, et al. 2020. Data from: Tolerant and intolerant macaques show different levels of structural complexity in their vocal communication. Dryad Digital Repository. ( 10.5061/dryad.905qfttgw) [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Rebout N, et al. 2020. Data from: Tolerant and intolerant macaques show different levels of structural complexity in their vocal communication. Dryad Digital Repository. ( 10.5061/dryad.905qfttgw) [DOI]
Supplementary Materials
Data Availability Statement
The dataset is available at the Dryad Digital Repository https://doi.org/10.5061/dryad.905qfttgw [83].