Figure 5. Uptake of Ub4ix by living cells and effect on ubiquitination.
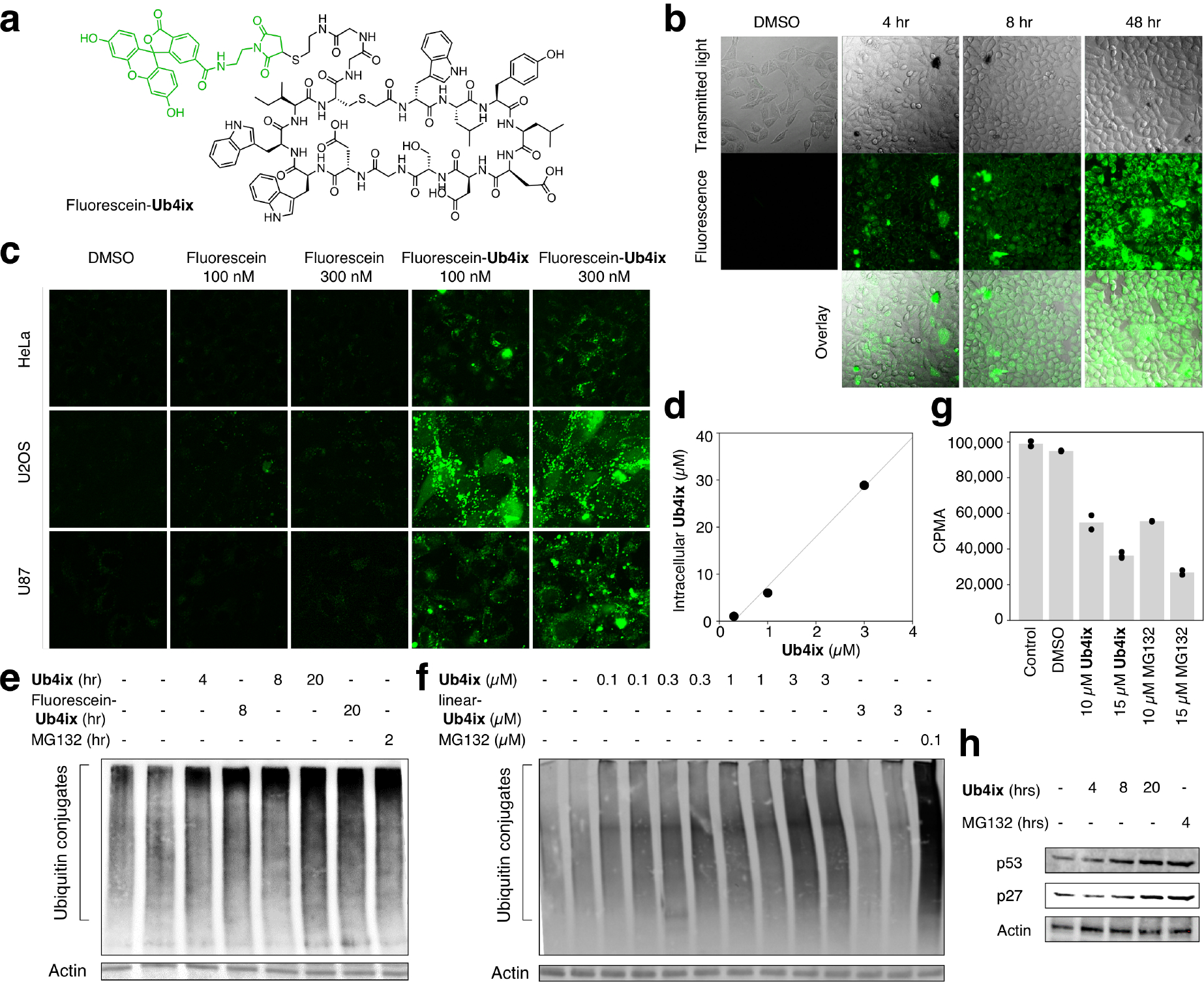
a) Fluorescein-Ub4ix structure. b) Ub4ix can enter cells. HeLa cells were incubated with fluorescein-Ub4ix cyclic peptide (20 μM). Internalization of the peptide into the cells was monitored at the indicated time points. c) In three cell lines, HeLa, U2OS osteosarcoma and U87 primary glioblastoma cells, fluorescein-Ub4ix is retained in cells after washing, whereas fluorescein alone is lost. d) Screening of the cellular uptake fluorescein-Ub4ix after incubation for 16 hr shows significant retention inside cells. e) Ub4ix induced the accumulation of Ub-conjugates. HeLa cells were incubated in the presence of Ub4ix, Fluorescein-Ub4ix, or MG132 for the indicated time-periods. f) Ub4ix induced the accumulation of Ub-conjugates in a dose dependent manner. Cells were lysed, resolved using SDS-PAGE, proteins were transferred to a nitrocellulose membrane, and the membrane was blotted using an antibody against Ub-conjugates and Actin. g) Ub4ix inhibits protein breakdown inside cells. HeLa cells were labeled with radioactive 35S-Met and 35S-Cys, and were then chased for 4 hr in “cold” media. Cells were either non-treated, or incubated in the presence of DMSO, Ub4ix, or MG132. Radioactivity levels (counts per min, CPMA) from protein breakdown during the chase phase, show that Ub4ix and MG132 inhibit. Bars represent mean values. h) Ub4ix causes the accumulation of proteins that are normally turned over by the UPS. HeLa cells were incubated in the presence of 10 μM Ub4ix or 10 μM MG132 for the indicated times. Cells were lysed, resolved using SDS-PAGE, proteins were transferred to a nitrocellulose membrane, and the membrane was blotted using an antibody against p53, p27 and Actin.
