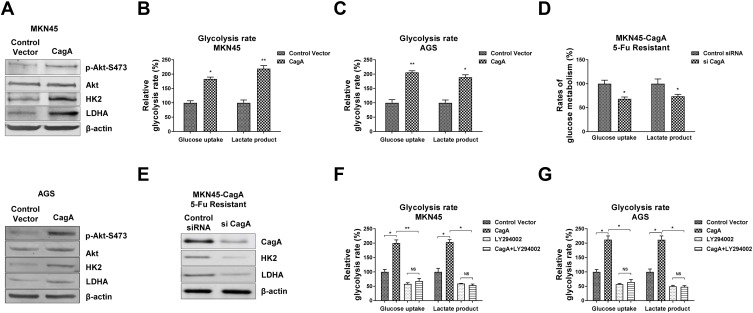
Figure 4.
CagA stimulates gastric cancer cell glycolysis through activating Akt. (A) MKN45 cells (upper) and AGS cells (lower) were transfected with control vector or CagA overexpression plasmid for 48 hours. The phosphorylation of Akt, total Akt, KH2 and LDHA were examined by Western blot. β-actin was a loading control. (B) MKN45 cells and (C) AGS cells were transfected with control vector or CagA overexpression plasmid for 48 hours. The glucose uptake and lactate product were measured. (D) MKN45-CagA 5-Fu resistant cells were transfected with control siRNA or siCagA for 48 hours, the glucose uptake, lactate product and (E) protein expressions of HK2 and LDHA were measured. (F) MKN45 cells and (G) AGS without or with CagA overexpression were treated with control or Akt inhibitor for 24 hours. The glucose uptake and lactate product were measured. Columns, mean of three independent experiments; bars, SE. NS, no significance; *p < 0.05; **p < 0.01.