Figure 2.
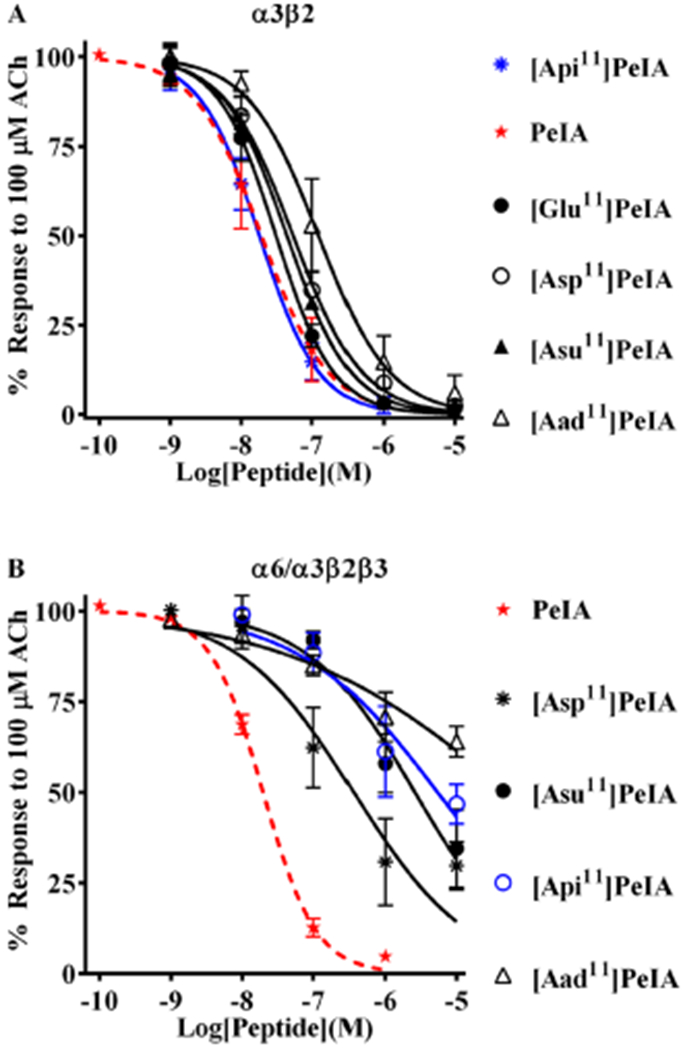
PeIA analogs with negatively charged amino acid residues in position 11 show increased selectivity for α3β2 nAChRs. Analogs with substitutions of Asn11 were synthesized and a concentration–response analysis was performed for each to determine the IC50 values. The value obtained for each analog was compared to that of the native peptide (red dashed curves) to assess the effects of the substitution. (A) Analogs with Asp, Glu, or Api substitutions showed <3-fold change in potency for α3β2 nAChRs. Aad, with a one-carbon longer side chain than Glu and one-carbon shorter than Api, reduced PeIA potency by 6-fold. (B) By contrast, all analogs showed substantially reduced potency (6- to 274-fold) for α6/α3β2β3 nAChRs. The most α3β-selective of these analogs was determined to be [Api11]PeIA (blue curve), which showed a 300-fold preference for the α3β2 subtype. A minimum of four oocytes was used for each IC50 determination, and the error bars indicate the SD. The analogs are listed in rank order of potency from top (most potent) to bottom (least potent); values are presented in Table 3.
