Figure 3.
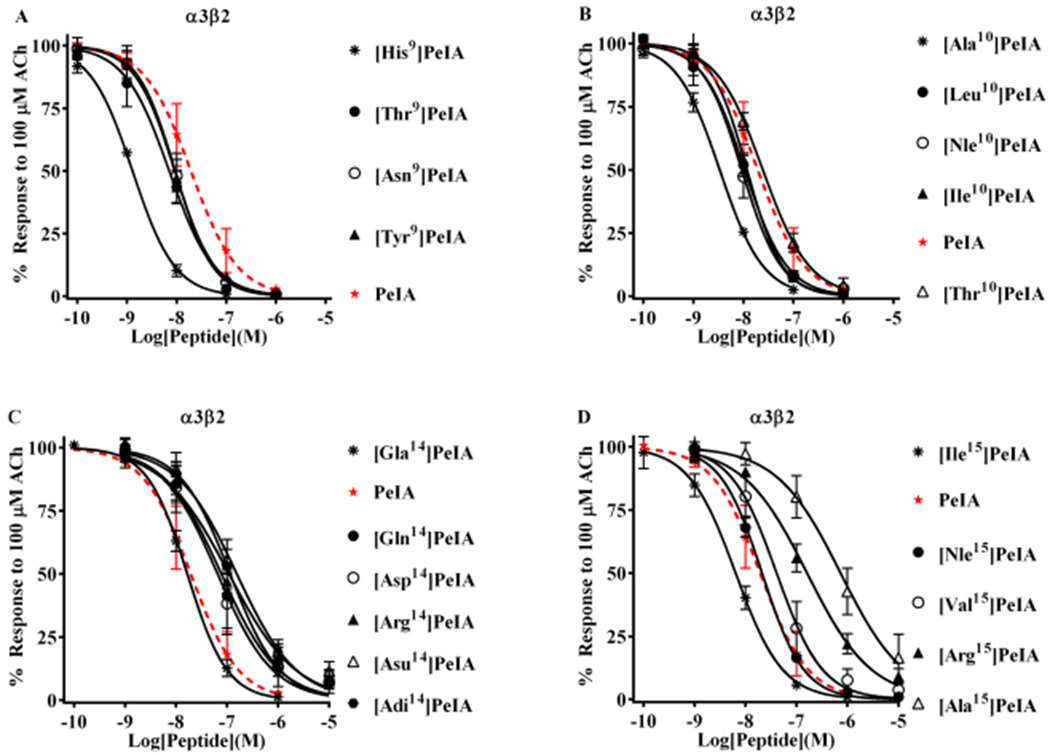
Structure–function analysis of PeIA identifies amino acid substitutions that increase potency for α3β2 nAChRs. PeIA analogs with substitutions of Ser9, Val10, Glu14, or Leu15 were synthesized and a concentration–response analysis was performed for each to determine the IC50 values. The value for each analog was compared to that of the native peptide to evaluate the interaction between the substituted positions and α3β2 nAChRs. (A) Analogs with Asn, Tyr, or Thr substitutions of Ser9 showed a <3-fold change in potency for α3β2 nAChRs. Substitution of Ser9 with His increased the potency of PeIA by 15-fold. (B) Substitution of Val10 with Ala increased (6-fold) PeIA potency, whereas other hydrophobic amino acids showed a <3-fold change but trended toward increased potency. Substitution with Thr also showed a <3-fold change. (C) Substitutions of Glu14 revealed the importance of a Glu in this position for PeIA potency on α3β2 nAChRs. Substitution with any other negatively charged amino acid reduced potency by 3- to 10-fold. A <3-fold change in potency was observed by changing Glu to Gla. Substitution of Glu14 with Gln or Arg reduced PeIA potency by 4- and 6-fold, respectively. (D) Varying effects on PeIA potency were observed when Leu15 was substituted with other hydrophobic amino acids, whereas Ile increased PeIA potency by 3-fold, Ala reduced potency by 38-fold; Nle and Val produced a <3-fold change. Arg reduced PeIA potency by 8-fold. A minimum of four oocytes was used for each IC50 determination and the error bars indicate the SD. The analogs are listed in rank order of potency from top (most potent) to bottom (least potent); values are provided in Table 4. Data for PeIA (red dashed curves) were previously presented in Figure 2 and shown for the ease of comparison to the curves of the analogs.
