Scheme 1. Synthesis of propofol analog 8.
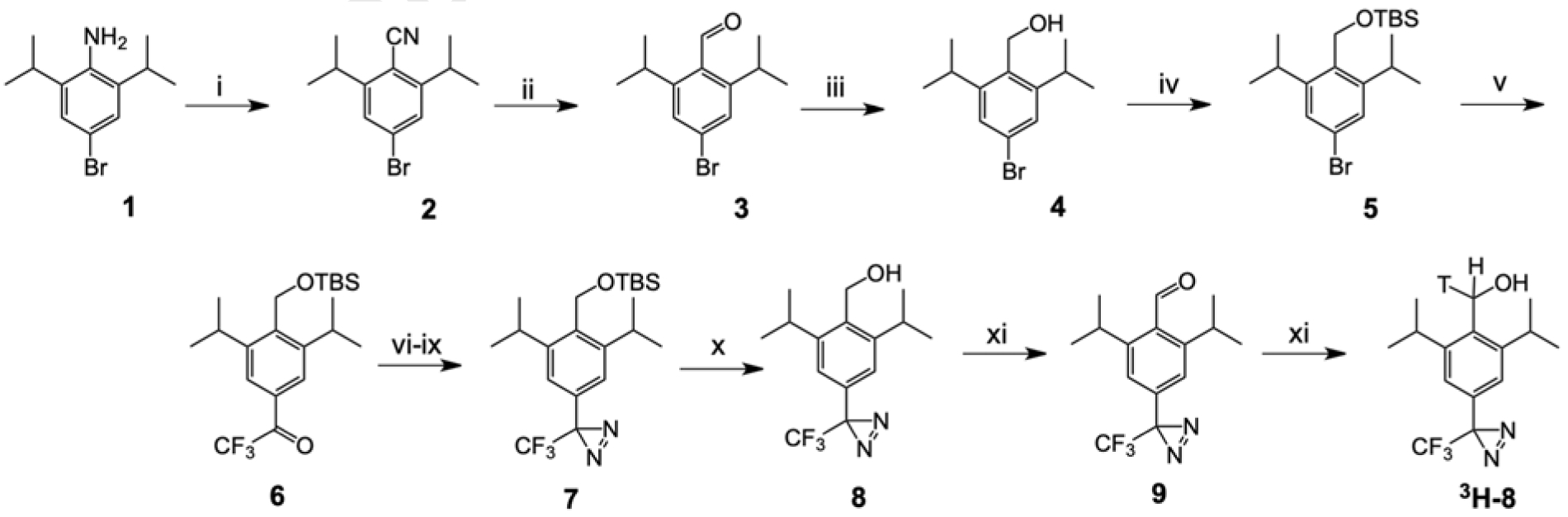
i: (a) HCl/NaNO2, 0°C; (b) CuCN-KCN, 70°C; ii: DIBAL-H; iii: NaBH4; iv: (a) t-butyldimethylsilyl chloride – 4-dimethylaminopyridine, 40°C; v: (a) n-BuLi, −78°C; (b) ethyl trifluoroacetate, −78°C; vi: hydroxylamine-HCl, 80°C; vii: tosyl chloride, 4-dimethylaminopyridine, diisopropylethylamine; viii: NH3/methanol; ix: I2, triethylamine; x: tetra-n-butylammonium fluoride,; xi: Dess-Martin periodinane; xii: NaB3H4.
