Table 1.
The pharmacological properties of the benzyl alcohol derivatives studied compared to propofol.
| Structure | Abbreviated name | LoRR in Tadpoles EC50 ± SD, μM (# of animals) | Enhancement of [3H]muscimol binding in α1β3γ2L GABAARs EC50 ± SD, μM (N) | Protection against photoincorporation in the β subunit of α1β3γ2L GABAARs in the presence of GABA | ||
|---|---|---|---|---|---|---|
| [3H]azietomidate IC50, μM) (N, R2) | [3H]pTFD-di-iPr-BnOH IC50, μM) (N, R2) | [3H]R-mTFD-MPAB IC50, μM (N, R2) | ||||
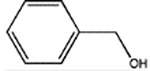 |
BnOH | 2,000 ± 210a | >50,000 | ND | ND | ND |
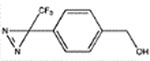 |
pTFD-BnOH | 28 ± 7b (30) | 700 ± 81 (3) | 305 ± 33 (5, 0.92) | ND | 465 ± 5 (5, 0.87) |
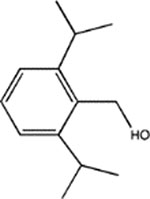 |
Di-iPr-BnOH | 16 ± 1 (30) | 40 ± 6 (2) | 92 ± 6 (5, 0.97) | ND | 243 ± 23 (5, 0.91) |
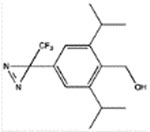 |
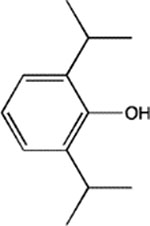
pTFD-di-iPr-BnOH | 2.5 ± 0.6 (73) | 7.4 ± 0.7 (6) | 460 ± 70 (4, 0.59) | 18 ± 2 (4, 0.94) | 26 ± 5 Bns = 42 ± 3% (4, 0.87) |
 |
Propofol | 0.63 ± 0.09c | 5.4 ± 0.9 (2) | 8 ± 1d | 32 ± 6 (4, 0.87) | 44 ± 4d |
