Abstract
Background: Sex-gender medicine focuses on differences and similarities in health and disease between men and women. The present study focused on the existence of male and female phenotypes when routine demographic, biochemical and haematological data are considered and aimed to determine the influence of smoking on phenotypes and evaluate the role of body weight on sex-gender differences in view of the fact that some of them can be utilized as biomarkers of diagnosis, diseases and therapeutic response. Methods: Eighty-five healthy young adult men (27 smokers and 58 non-smokers) and 85 women (32 smokers and 53 non-smokers) well matched for age were enrolled. 31 haematochemical parameters were measured and data were analysed before and after normalization for body weight applying the two-way analysis of variance and principal component analysis (PCA). Results: In non-smoking cohorts, there were numerous sex-gender differences and PCA analysis distinguished two different phenotypes: males and females. Body weight normalization induced qualitatively and quantitatively changes, but male and female phenotypes were still well evident. Smoking influenced numerous parameters and PCA analysis evidenced that these changes led to the abolition of male and female phenotypes. Conclusions: Personalized medicine has the goals to study modification of markers profile in the single individual, which is strongly influenced by sex-gender and smoking habit. In non-smokers, male and female phenotypes are present independently from the quantitation method used. In smokers only one phenotype is present. These results suggest that smoking and sex-gender should be considered as an independent variable in clinical research.
Keywords: Sex-gender differences, smoking habit, body weight, phenotype
Introduction
Sex and gender* play a crucial role in health, yet they are often neglected in health care [1]. However, research on sex and gender highlights that several diseases and risk factors affect men and women differently such as pharmacological therapies [1-3]. Sex differences can be associated with body weight, which is usually lower in women than in men [4]; however, some researchers disagree [5,6]. Actually, few studies have been performed on absolute and normalized (by body weight) concentrations of biomarker performance [7-9]. To date, there is no consensus on how they could be adopted in routine clinical practice.
Being a woman or man depends on complex interactions of female and male bodies with the surrounding environment; and smoking is one of the most relevant environmental factors that influences human health [10,11] due to its prevalence (> 1 billion smokers worldwide) [12] and negative impact on health [10,11]. Tobacco smoking is a risk factor for premature mortality being also a contributor to ethnic and socioeconomic inequalities in mortality [13]. In western countries, it is responsible for most of the excess mortality of poor men. This point is less clear in women [14].
Some reports show the role of sex-gender in the pathobiology of tobacco [15], with women being more susceptible than men versus the negative effect on health [16-18]. Further, smoking can affect pharmacokinetic and pharmacodynamic profiles of numerous drugs, including cyclosporine, sexual hormones [19-23] and this may reflect on therapeutic responses. Indeed, for oral hormonal contraceptives users, a Food Drug Administration warning says “women are strongly advised not to smoke” [24].
Evaluating sex-gender differences in routine biochemical and haematological phenotypes will be crucial in the development of a personalized medicine encompassing sex and gender sensitive strategies for prevention, diagnosis and treatment. Thus, the goal of this work is to investigate whether the sex and gender and the smoking status of healthy young adults could affect both routine biochemical and haematological parameters to evaluate potential male and female phenotypes and describing the influence of body dimension on sex and gender differences.
* The concept of sex includes the biological differences between men and women, whereas gender includes psychological, social and cultural characteristics (including lifestyle and behavioural habits, and therefore smoking). However, they interact [25-27], therefore in order to emphasize the importance of such interaction, the term sex-gender will be used throughout the text.
Methods
Cohort’s characteristics
A total of 85 healthy men and 85 healthy women with regular menstrual cycles (28 days) both aged between 18 and 40 years, were enrolled during a voluntary blood donation in Sassari, Italy. All participants were informed that an aliquot of blood would be kept for the study, and all procedures were conducted in accordance with the Helsinki Declaration. The inclusion and exclusion criteria were already described in Campesi 2013 [28]. Briefly, healthy men and women were included. All the participants were free of kidney, liver, heart, endocrine diseases and infectious disease for at least 2 months before their participation. None of the subjects was on long-term medications, including oral contraceptives for women. Women were all evaluated during the follicular phase (1-10 days) in view of the fact biomarkers are influenced by endogenous and exogenous sexual hormones [29,30]. Smokers (27 men and 32 women) were defined as subjects who smoked at least one cigarette per day at the time of blood collection. All the information was collected through a questionnaire given to the volunteer at the time of the donation.
Biochemical and hematological examinations
Laboratory assessments were conducted, immediately after collection, on 10 mL samples of fasting blood (collected at 8.00 am and 10.00 am; silicone coating for serum determinations and potassium-ethylenediaminetetra-acetic acid for other assessments). Plasma and/or serum aliquots were used to test for glycaemia, total cholesterol (TChol), low (LDL) and high (HDL) density lipoprotein, HDL/LDL, triglycerides (TG), aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ-glutamyl transpeptidase (γ-GT), bilirubin, alkaline phosphatase, creatinine (Cr), urea, uric acid, Ca2+, Na+, K+, sideremia, and thyroid-stimulating hormone (TSH). Full blood aliquots were used to measure red cell (RBC) and white blood count (WBC), leucocyte formula, haemoglobin (Hb), haematocrit, and mean corpuscular volume (MCV), and platelet count (PLT). These factors were measured following procedures described in Campesi et al. (2012). Creatinine clearance (CrCl) was calculated using the formula [140 - age (year)] * weight (kg)]/[72 * serum Cr (mg/dl)] for men and multiplying by 0.85 for women.
Statistical analysis
Data are reported as the means ± SD before and after normalization for body weight. The distribution of samples was evaluated using the Kolmogorov-Smirnov and Shapiro tests. Univariate analysis of blood parameters was performed by Two-Way Analysis of Variance followed by Pairwise Multiple Comparison Procedures to analyse the effect of sex and gender and smoking using Sigma-Stat 3.1 software (Systat Software, Erkrath, Germany). A P-value < 0.05 was considered statistically significant. Adequate total sample size for Two way ANOVA was calculated using G*Power software (free) based on 95% confidence and 80% (effect size as medium (f = 0.25), number of groups (4) and numerator degree of freedom as 1 [31].
PCA was performed using the correlation matrix of standardized variables, by smoking status and sex-gender. Components with eigenvalues greater than 1.00 were retained for further analysis. Associations between the data and components were established using varimax-rotated factor loadings. PCA was performed using IBM SPSS 23 (IBM Armonk, USA).
Results
Characteristics of cohorts
Cohorts were well matched for age. In particular, the age (years) of male smokers and non-smokers were 28.4 ± 4.6 (n = 27) and 25.9 ± 4.9 (n = 58), respectively; whereas the age (years) of female smokers and non-smokers 28.2 ± 4.8 (n = 32) and 27.7 ± 5.3 (n = 53), respectively without significant differences between sexes.
Smokers of both sexes had a lower educational level in comparison with non-smokers with not significant differences between men and women. In particular, 69.4% and 65.6% of male and female smokers had the lower education levels, respectively.
On average, body weight (kg) was significantly different between male and female non-smokers being 74.4 ± 10.4 versus 53.7 ± 7.0 (P < 0.001) respectively; and 69.6 ± 10.6 versus 56.0 ± 8.4 (P < 0.001) between male and female smokers, respectively. On average, body mass index (BMI) (kg/m2) was significantly higher in non-smoking men (24.1 ± 2.4) than in non-smoking women (20.7 ± 2.5; P < 0.001). BMI was also significantly higher in smoking men (22.8 ± 3.1) than in smoking women (21.3 ± 3.3; P = 0.047). The statistically significant differences in body dimension between sex-genders within each group suggested us to perform the subsequent analyses using the absolute values and the values obtained after normalization for body weight.
Baseline data
The Two Way Analysis of Variance demonstrated that, both before and after body weight normalisation, some parameters were affected only by sex-gender, others only by the smoke, and in others it was observed an interaction between sex-gender and smoking (Tables S1, S2).
Effect of sex-gender and smoking on biochemical and haematological parameters before body weight normalization
Independently from cigarette smoking, sex-gender affected 14 parameters. Men had higher glycaemia, TG, Cr, CrCl, urea, AST, γ-GT, Na+, RBC, haematocrit, and monocytes. TChol, HDL, and eosinophils count were significantly lower in men (Table 1), while non-statistically significant sex-gender effect was detected for all the other measured parameters (LDL, HDL/LDL, bilirubin, Ca2+, K+, sideremia, TSH, MCV, WBC, neutrophils, lymphocytes, and basophils) (Table 1).
Table 1.
Biochemical and haematological parameters stratified by sex-gender and smoking habit before body weight normalization
| 2-way ANOVA P value | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Parameters | Groups | Men | Women | Sex-gender | Smoke | Interaction |
| Glycaemia (mg dl-1) | Non-S | 80.9 ± 9.1 | 77.3 ± 9.7 | 0.011 | ns | ns |
| S | 78.5 ± 8.6 | 73.9 ± 12.6 | ||||
| TChol (mg dl-1) | Non-S | 181.4 ± 34.8 | 182.8 ± 28.7 | 0.037 | ns | ns |
| S | 166.0 ± 38.0 | 186.5 ± 27.1 | ||||
| LDL (mg dl-1) | Non-S | 111.1 ± 29.6 | 109.2 ± 24.3 | ns | 0.032 | ns |
| S | 93.3 ± 29.6 | 108.2 ± 21.3 | ||||
| HDL (mg dl-1) | Non-S | 52.4 ± 12.1 | 60.7 ± 11.4 | < 0.001 | ns | ns |
| S | 54.3 ± 13.9 | 61.3 ± 11.0 | ||||
| HDL/LDL | Non-S | 0.5 ± 0.2a2 | 0.6 ± 0.2 | ns | 0.043 | 0.047 |
| S | 0.7 ± 0.2 | 0.6 ± 0.2 | ||||
| TG (mg dl-1) | Non-S | 89.4 ± 38.5 | 66.8 ± 21.8 | 0.036 | ns | ns |
| S | 91.6 ± 64.3 | 87.2 ± 37.2 | ||||
| Cr (mg ml-1) | Non-S | 0.9 ± 0.1 | 0.7 ± 0.1 | < 0.001 | ns | ns |
| S | 0.8 ± 0.1 | 0.7 ± 0.1 | ||||
| CrCl (mg min-1) | Non-S | 120.2 ± 2 1.7 | 100.6 ± 16.3 | < 0.001 | ns | ns |
| S | 114.6 ± 26.2 | 104.1 ± 16.2 | ||||
| Urea (mg dl-1) | Non-S | 35.5 ± 7.7 | 28.8 ± 8.6 | < 0.001 | ns | ns |
| S | 32.8 ± 8.3 | 27.9 ± 7.3 | ||||
| Uric Acid (mg dl-1) | Non-S | 5.4 ± 1.1a1,d3 | 3.5 ± 0.9 | < 0.001 | ns | 0.041 |
| S | 4.9 ± 1.2c2 | 3.7 ± 0.9 | ||||
| AST (U l-1) | Non-S | 27.9 ± 13.5 | 19.5 ± 5.0 | < 0.001 | ns | ns |
| S | 24.2 ± 9.9 | 20.0 ± 5.3 | ||||
| ALT (U l-1) | Non-S | 35.2 ± 19.8a2,d3 | 17.8 ± 7.9 | < 0.001 | ns | 0.007 |
| S | 25.4 ± 11.4 | 20.4 ± 11.5 | ||||
| γ-GT (U l-1) | Non-S | 25.6 ± 11.7 | 15.3 ± 4.0 | < 0.001 | ns | ns |
| S | 24.9 ± 13.5 | 19.4 ± 15.8 | ||||
| Bilirubin (mg dl-1) | Non-S | 0.8 ± 0.4 | 0.7 ± 0.4 | ns | ns | ns |
| S | 0.7 ± 0.4 | 0.6 ± 0.4 | ||||
| Alkaline Phosphatase (U l-1) | Non-S | 69.8 ± 15.5d3 | 58.6 ± 14.5 | 0.014 | ns | 0.034 |
| S | 63.4 ± 15.1 | 62.5 ± 14.0 | ||||
| Na+ (mEq l-1) | Non-S | 141.9 ± 2.6 | 140.1 ± 2.1 | < 0.001 | ns | ns |
| S | 141.5 ± 1.8 | 139.7 ± 2.4 | ||||
| K+ (mEq l-1) | Non-S | 4.2 ± 0.3 | 4.0 ± 0.3 | ns | ns | ns |
| S | 4.2 ± 0.4 | 4.1 ± 0.3 | ||||
| Ca2+ (mg dl-1) | Non-S | 9.4 ± 0.4 | 9.4 ± 0.4 | ns | ns | ns |
| S | 9.5 ± 0.4 | 9.6 ± 0.4 | ||||
| Sideremia (µg l-1) | Non-S | 102.2 ± 38.2 | 84.0 ± 38.1 | ns | ns | ns |
| S | 90.1 ± 25.7 | 89.3 ± 44.4 | ||||
| TSH (µUI ml-1) | Non-S | 2.0 ± 1.1 | 1.9 ± 0.9 | ns | ns | ns |
| S | 2.0 ± 1.1 | 1.8 ± 0.9 | ||||
| RBC (1012 l-1) | Non-S | 5.6 ± 0.6 | 4.8 ± 0.5 | < 0.001 | ns | ns |
| S | 5.5 ± 0.7 | 4.7 ± 0.4 | ||||
| Hb (g dl-1) | Non-S | 14.5 ± 1.2d3 | 12.5 ± 1.2 | < 0.001 | ns | 0.031 |
| S | 14.1 ± 1.3c3 | 12.9 ± 0.9 | ||||
| Haematocrit (%) | Non-S | 43.6 ± 2.8 | 38.7 ± 3.3 | < 0.001 | ns | ns |
| S | 42.7 ± 2.8 | 39.2 ± 2.9 | ||||
| MCV (fl) | Non-S | 78.1 ± 11.5 | 80.2 ± 8.2 | ns | ns | ns |
| S | 79.5 ± 12.1 | 83.4 ± 7.0 | ||||
| WBC (109 l-1) | Non-S | 6.8 ± 1.4 | 6.3 ± 1.5 | ns | < 0.001 | ns |
| S | 7.6 ± 1.4 | 7.3 ± 1.7 | ||||
| Neutrophils (103 µl-1) | Non-S | 3.6 ± 1.0 | 3.4 ± 1.1 | ns | 0.004 | ns |
| S | 4.2 ± 1.1 | 3.9 ± 1.2 | ||||
| Lymphocytes (103 µl-1) | Non-S | 2.3 ± 0.6 | 2.2 ± 0.6 | ns | 0.008 | ns |
| S | 2.5 ± 0.8 | 2.6 ± 0.8 | ||||
| Monocytes (103 µl-1) | Non-S | 0.4 ± 0.1 | 0.3 ± 0.10b1,c1 | < 0.001 | 0.008 | ns |
| S | 0.5 ± 0.1d3 | 0.4 ± 0.12 | ||||
| Eosinophils (103 µl-1) | Non-S | 0.2 ± 0.1 | 0.4 ± 0.26 | 0.020 | 0.04 | ns |
| S | 0.2 ± 0.1 | 0.2 ± 0.10 | ||||
| Basophils (103 µl-1) | Non-S | 0.04 ± 0.03 | 0.03 ± 0.02 | ns | ns | ns |
| S | 0.06 ± 0.05 | 0.04 ± 0.03 | ||||
| PLT (109 l-1) | Non-S | 259.8 ± 48.8 | 273.3 ± 53.8b2 | < 0.001 | Ns | 0.006 |
| S | 246.4 ± 50.1c3 | 305.6 ± 50.1 | ||||
Data are reported as the means ± SD of 58 male non-smokers, 27 male smokers, 53 female non-smokers and 32 female smokers. P-values are reported for sex and smoking effects, as well as for sex x smoking interaction. For each parameter, the degree of freedom was 1. Multiple comparison analysis: a1-b1-c1-d1 for P = 0.05-0.01; a2-b2-c2-d2 for P = 0.01-0.001; a3-b3-c3-d3 for P ≤ 0.001; S = smoking; Non-S = non-smoking; ns = not significant.
Independently from sex, smoking affected 6 parameters. Smokers had lower levels of LDL and higher count of WBC, neutrophils, and lymphocytes (Table 1). The count of monocytes and eosinophils was globally higher in smokers than in non-smokers. Moreover, smoker women had higher count of monocytes than non-smoker ones. Smoking effect was not detected for all the other parameters (glycaemia, TChol, HDL, TG, Cr, CrCl, urea, AST, γ-GT, bilirubin, alkaline phosphatase activity, Na+, Ca2+, K+, sideremia, TSH, RBC, haematocrit, MCV, basophils) (Table 1).
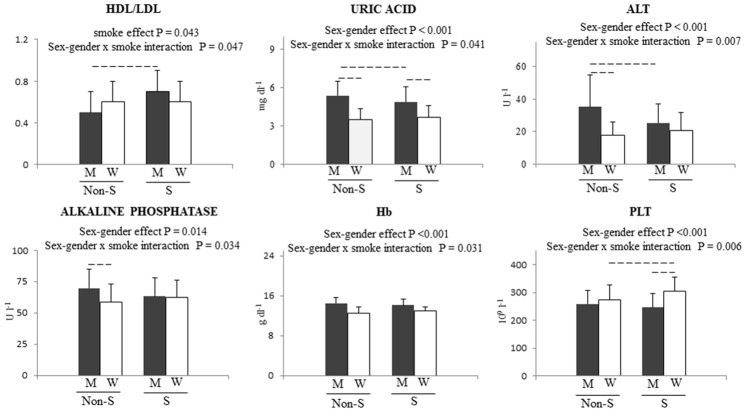
A statistically significant interaction between sex-gender and smoking was detected for 6 parameters (Table 1; Figure 1). HDL/LDL ratio was significantly higher only in male smokers, while smoking females had significantly higher PLT count than smoking men and non-smoking women (Table 1; Figure 1). Uric acid significantly differed between male non-smokers and smokers, between male smokers and female smokers, and between male non-smokers and female non-smokers, with the highest value detected in male non-smokers. The highest levels of ALT were found in male non-smokers versus non-smoking men and women (Table 1; Figure 1). Alkaline phosphatase activity differed only in non-smokers, being higher in non-smoking men. As expected, Hb was higher in males than in females, irrespective of smoking (Table 1; Figure 1). Finally, bilirubin, TSH, K+, Ca++, sideremia, MCV and basophils were not affected by smoking and sex-gender.
Figure 1.
Sex-gender x smoking interactions before normalization for body weight. P values are reported for main effect and for interaction. Dashed lines represent a P < 0.05 for multiple comparison test. W = women, M = men, S = smokers, Non S = non smokers.
Effect of sex-gender and smoking after normalization for body weight
After body weight normalization, independently from cigarette smoking, sex-gender influenced 13 parameters. Weight-corrected TChol, LDL, HDL, alkaline phosphatase activity, K+, Ca++ sideraemia, TSH, RBC and PLT counts, Hb, haematocrit, and MCV were significantly lower in men than in women (Table 2). The following parameters: TG, Cr, urea, uric acid, AST, γ-GT, bilirubin, monocytes, eosinophils and basophils did not vary significantly.
Table 2.
Biochemical and haematological parameters stratified by sex-gender and smoking habits after body weight normalization
| 2-way ANOVA P value | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Parameters | Groups | Men | Women | Sex-gender | Smoke | Interaction |
| Glycaemia (mg dl-1) | Non-S | 1.1 ± 0.2 | 1.5 ± 0.3b1,d2 | < 0.001 | ns | 0.033 |
| S | 1.1 ± 0.2c2 | 1.3 ± 0.3 | ||||
| TChol (mg dl-1) | Non-S | 2.5 ± 0.5d3 | 3.4 ± 0.6 | < 0.001 | ns | ns |
| S | 2.4 ± 0.5c3 | 3.4 ± 0.5 | ||||
| LDL (mg dl-1) | Non-S | 1.5 ± 0.4d3 | 2.0 ± 0.4 | < 0.001 | ns | ns |
| S | 1.3 ± 0.4c3 | 2.0 ± 0.4 | ||||
| HDL (mg dl-1) | Non-S | 0.7 ± 0.2d3 | 1.1 ± 0.3 | < 0.001 | ns | ns |
| S | 0.8 ± 0.2c3 | 1.1 ± 0.2 | ||||
| HDL/LDL | Non-S | 0.007 ± 0.004a2,d3 | 0.01 ± 0.004 | < 0.001 | ns | 0.014 |
| S | 0.01 ± 0.005 | 0.01 ± 0.003 | ||||
| TG (mg dl-1) | Non-S | 1.2 ± 0.5 | 1.3 ± 0.4b2 | ns | 0.023 | ns |
| S | 1.3 ± 0.8c1 | 1.6 ± 0.8 | ||||
| Cr (mg ml-1) | Non-S | 0.01 ± 0.001 | 0.01 ± 0.002 | ns | ns | ns |
| S | 0.01 ± 0.003 | 0.01 ± 0.001 | ||||
| Urea (mg dl-1) | Non-S | 0.49 ± 0.13 | 0.55 ± 0.18 | ns | ns | ns |
| S | 0.48 ± 0.14 | 0.51 ± 0.15 | ||||
| Uric Acid (mg dl-1) | Non-S | 0.07 ± 0.01 | 0.07 ± 0.02 | ns | ns | ns |
| S | 0.07 ± 0.02 | 0.07 ± 0.02 | ||||
| AST (U l-1) | Non-S | 0.38 ± 0.15 | 0.37 ± 0.11 | ns | ns | ns |
| S | 0.36 ± 0.17 | 0.36 ± 0.10 | ||||
| ALT (U l-1) | Non-S | 0.47 ± 0.25a1,d3 | 0.33 ± 0.15 | 0.027 | ns | 0.037 |
| S | 0.37 ± 0.17 | 0.36 ± 0.19 | ||||
| γ-GT (U l-1) | Non-S | 0.35 ± 0.15 | 0.29 ± 0.09 | ns | 0.048 | ns |
| S | 0.37 ± 0.18 | 0.35 ± 0.26 | ||||
| Bilirubin (mg dl-1) | Non-S | 0.01 ± 0.006 | 0.01 ± 0.009 | ns | ns | ns |
| S | 0.01 ± 0.006 | 0.01 ± 0.008 | ||||
| Alkaline Phosphatase (U l-1) | Non-S | 0.96 ± 0.27d2 | 1.11 ± 0.36 | < 0.001 | ns | ns |
| S | 0.93 ± 0.26c2 | 1.13 ± 0.27 | ||||
| Na+ (mEq l-1) | Non-S | 1.94 ± 0.26d3 | 2.65 ± 0.36 | < 0.001 | ns | ns |
| S | 2.09 ± 0.32c3 | 2.55 ± 0.38 | ||||
| K+ (mEq l-1) | Non-S | 0.06 ± 0.009d3 | 0.08 ± 0.01 | < 0.001 | ns | ns |
| S | 0.06 ± 0.009c3 | 0.07 ± 0.01 | ||||
| Ca2+ (mg dl-1) | Non-S | 0.13 ± 0.02d3 | 0.18 ± 0.03 | < 0.001 | ns | ns |
| S | 0.14 ± 0.02c3 | 0.17 ± 0.02 | ||||
| Sideremia (µg l-1) | Non-S | 1.42 ± 0.58 | 1.59 ± 0.78 | 0.04 | ns | ns |
| S | 1.34 ± 0.45 | 1.64 ± 0.82 | ||||
| TSH (µUI ml-1) | Non-S | 0.03 ± 0.02d2 | 0.04 ± 0.01 | 0.026 | ns | ns |
| S | 0.03 ± 0.02 | 0.03 ± 0.02 | ||||
| RBC (1012 l-1) | Non-S | 0.08 ± 0.01d3 | 0.09 ± 0.01 | < 0.001 | ns | ns |
| S | 0.08 ± 0.02 | 0.09 ± 0.01 | ||||
| Hb (g dl-1) | Non-S | 0.19 ± 0.03d3 | 0.24 ± 0.03 | < 0.001 | ns | ns |
| S | 0.21 ± 0.03c2 | 0.24 ± 0.04 | ||||
| Haematocrit (%) | Non-S | 0.59 ± 0.08d3 | 0.73 ± 0.11 | < 0.001 | ns | ns |
| S | 0.63 ± 0.09c2 | 0.71 ± 0.11 | ||||
| MCV (fl) | Non-S | 1.07 ± 0.19d3 | 1.52 ± 0.26 | < 0.001 | ns | ns |
| S | 1.16 ± 0.22c3 | 1.52 ± 0.26 | ||||
| WBC (109 l-1) | Non-S | 0.09 ± 0.02a1,d3 | 0.12 ± 0.03b1 | < 0.001 | < 0.001 | ns |
| S | 0.11 ± 0.02c2 | 0.13 ± 0.04 | ||||
| Neutrophils (103 µl-1) | Non-S | 0.05 ± 0.01a1,d3 | 0.06 ± 0.02 | < 0.001 | 0.006 | ns |
| S | 0.06 ± 0.02 | 0.07 ± 0.02 | ||||
| Lymphocytes (103 µl-1) | Non-S | 0.03 ± 0.009d3 | 0.04 ± 0.01b1 | < 0.001 | 0.005 | ns |
| S | 0.04 ± 0.01c2 | 0.05 ± 0.02 | ||||
| Monocytes (103 µl-1) | Non-S | 0.006 ± 0.002 | 0.006 ± 0.002b1 | ns | 0.005 | ns |
| S | 0.007 ± 0.002 | 0.007 ± 0.002 | ||||
| Eosinophils (103 µl-1) | Non-S | 0.003 ± 0.002a2 | 0.003 ± 0.002 | ns | 0.005 | ns |
| S | 0.005 ± 0.008 | 0.004 ± 0.002 | ||||
| Basophils (103 µl-1) | Non-S | 0.0006 ± 0.0005 | 0.0006 ± 0.0007 | ns | ns | ns |
| S | 0.0009 ± 0.0008 | 0.0008 ± 0.0007 | ||||
| PLT (109 l-1) | Non-S | 3.58 ± 0.89d3 | 5.17 ± 1.24 | < 0.001 | ns | ns |
| S | 3.61 ± 0.92c3 | 5.59 ± 1.37 | ||||
Data are reported as the means ± SD. P-values are reported for sex-gender and smoking effects, as well as for sex-gender x smoking interaction. For each parameter, the degree of freedom was 1. Multiple comparison analysis: a1-b1-c1-d1 for P = 0.05-0.01; a2-b2-c2-d2 for P = 0.01-0.001; a3-b3-c3-d3 for P ≤ 0.001; S = smoking; Non-S = non-smoking, ns = not significant.
After body weight normalisation and independently from sex-gender, smoking influenced 4 parameters (Table 2). Female smokers had higher levels of TG and monocytes than female non-smokers did, while eosinophils and γ-GT were significantly higher in male smokers than in non-smokers (Table 2). The following parameters: TChol, LDL, HDL, Cr, urea, uric acid, AST, bilirubin, alkaline phosphatase activity, K+, Ca++, sideremia, TSH, RBC, Hb, haematocrit, MCV, basophils and PLT did not vary significantly.
After body weight normalization, 4 parameters (glycaemia, HDL/LDL ratio, ALT, Na+) showed a statistically significant interaction between sex-gender and smoking (Table 2; Figure 2). Female non-smokers had the highest value of glycaemia, which differed from those of smokers of both sexes. Moreover, in male smokers, glycaemia was significantly higher than in female smokers (Table 2; Figure 2). HDL/LDL ratio showed a difference between male non-smokers and male smokers, and between male non-smokers and female non-smokers, with the lowest value detected in male non-smokers (Table 2; Figure 2). Male non-smokers had significantly higher levels of ALT in comparison with female non-smokers and male smokers (Table 2; Figure 2). Finally, females had significantly higher Na+ in comparison with their male counterparts (Table 2; Figure 2).
Figure 2.
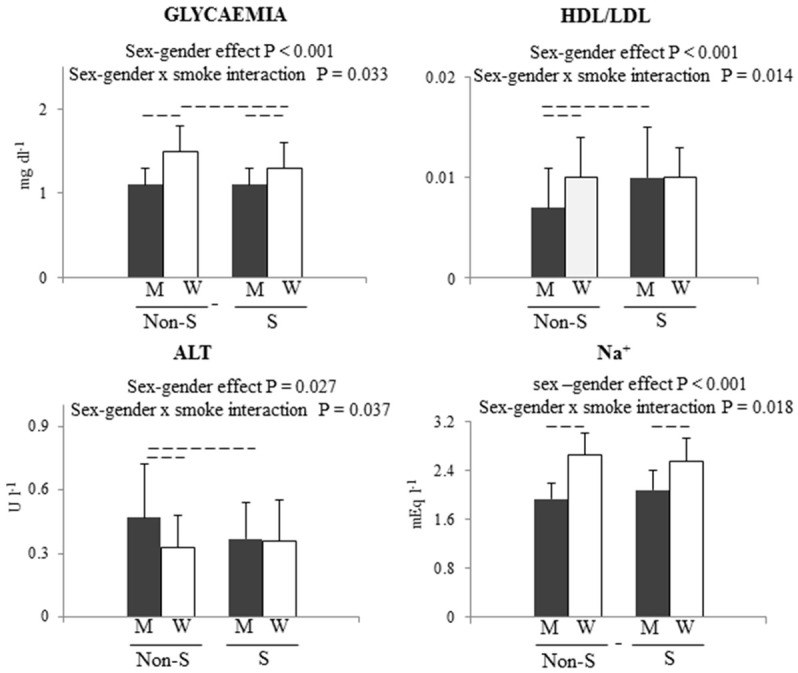
Sex-gender x smoking interactions after normalization for body weight. P values are reported for main effect and for interaction. Dashed lines represent a P < 0.05 for multiple comparison test. W = women, M = men, S = smokers, Non S = non smokers.
Comparison between Tables 1 and 2
Before adjustment for body weight, independently from smoking, men had higher glycaemia, alkaline phosphatase activity, Na+, RCB, haematocrit, which became significantly higher in women than in men after body weight normalization. Interestingly, TChol, HDL, and PLT were significantly higher in women than in men before and after body weight normalization. The body weight normalization induced a loss of statistical significance for TG, Cr, urea, uric acid, AST, γ-GT, eosinophils and monocytes whereas other parameters such as LDL, HDL/LDL, K+, Ca2+, sideremia, TSH, MCV, WBC, neutrophils, and lymphocytes became statistically significant being higher in women than in men.
Before body weight correction, HDL/LDL, uric acid, ALT, alkaline phosphatase, Hb, PLT showed a statistically significant interaction between sex-gender and smoking. The body weight normalisation reduced them from 6 to 4 leaving unchanged the interactions of HDL/LDL and ALT. The body weight normalisation also changed the parameters that were not influenced by smoking and sex-gender before normalisation.
PCA in smokers and non-smokers before and after body weight normalization
PCA analysis (a statistical procedure that converts a set of observations of possibly correlated variables into a set of values of linearly uncorrelated variables) was conducted using all analytic data, body weight, BMI and age. PCA analysis clearly identified male and female phenotypes (P = 0.035) (Figure 3A) after the stratification of all data only for sex. Male and female phenotypes (P = 0.046) were still present when PCA was applied on non-smoking cohorts (Figure 3C). After normalization for body weight, PCA analysis of all data still identified male and female groups, even if PCA score (Figure 4A, 4C) was not statistically different between sexes (P > 0.05). After normalization for body weight, male and female phenotypes were still present when PCA was applied on non-smoking cohorts (Figure 4B, 4D). Surprisingly, the PCA analysis performed stratifying all data for tobacco smoking habit evidenced only one phenotype (Figure 3B) (P = 0.094). The very same results (P = 0.080) were obtained when PCA was applied to smoking cohort of both genders (Figure 3D).
Figure 3.
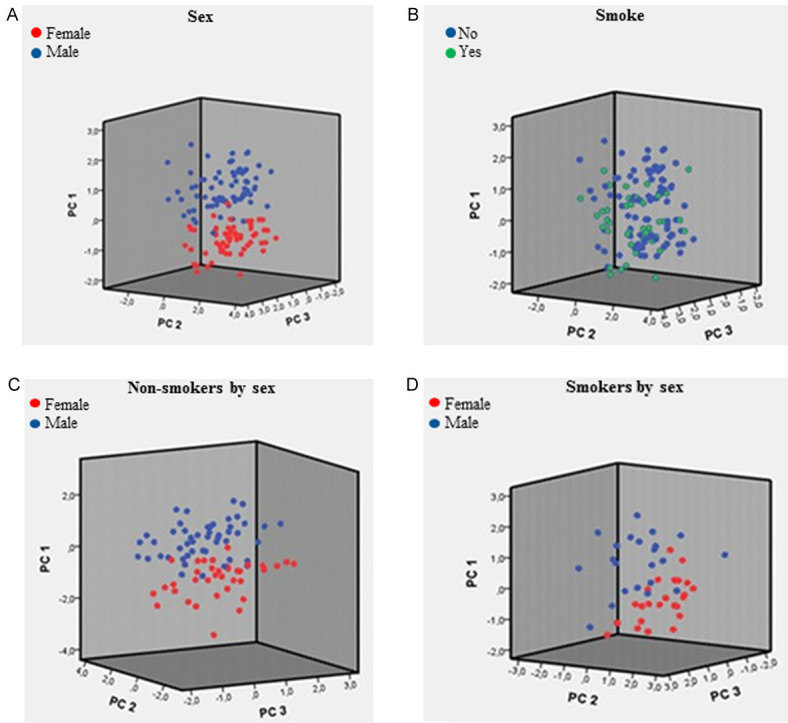
A. PCA score by sex-gender (N = 134); B. PCA score by smoking habit (N = 132); C. PCA score by sex-gender (non-smokers subjects, N = 86); D. PCA score by sex-gender (smoker subjects, N = 46).
Figure 4.
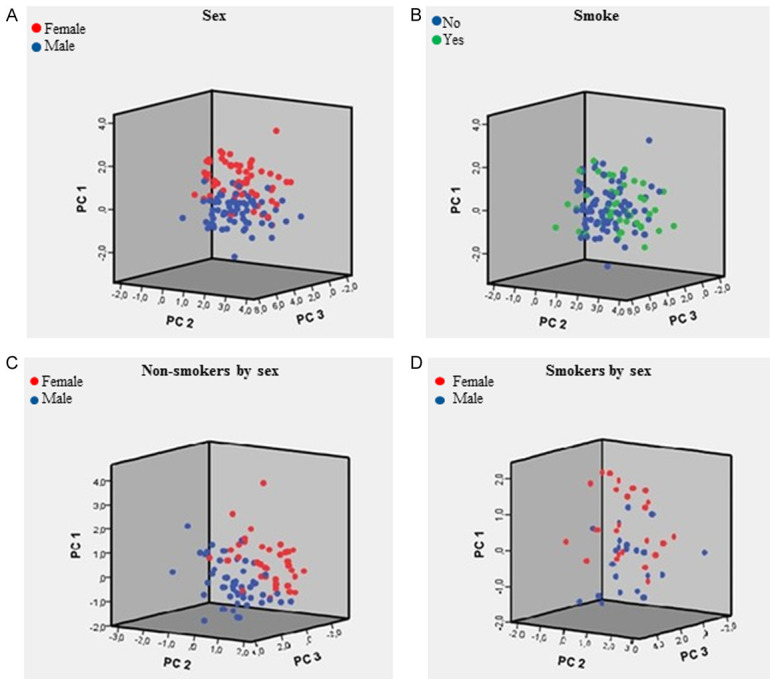
PCA obtained after data normalization by weight. A. PCA score by sex-gender (N = 134); B. PCA score by smoking habit (N = 132); C. PCA score by sex-gender (non-smokers subjects, N = 86); D. PCA score by sex-gender (smoker subjects, N = 46).
Before normalization of body weight, in female and male smokers, the first two principal components (PCs) explained 29.2% and 32.8% of the variance, respectively (Figure S1A, S1B). In female and male non-smokers, the first two PCs explained 27.4% and 27.3% of the variance, respectively (Figure S1C, S1D). Before normalization of body weight, in females who smoke, the variables with the highest loading on PC1 were WBC, monocytes, neutrophils, lymphocytes, eosinophils, glycaemia, and K+ (Figure S1A). Before normalization of body weight, in males who smoke, the most important variables for PC1 were TChol, TG, LDL, age, weight and BMI (Figure S1B). In non-smokers, the profile of variables was more similar between males and females being WBC, lymphocytes, monocytes, neutrophils, eosinophils and PLT were positively related to PC1, and TG, glycaemia, TChol, whereas LDL being negatively related to PC1 (Figure S1C, S1D).
After body weight normalization, in female smokers, minerals, RBC, Hb, haematocrit, MCV, PLT, lymphocytes and Cr were variables with the highest loading on PC1 (Figure S1E). Whereas, after body weight normalization, in male smokers (Figure S1F), PC1 was similar, with minerals, RBC, haematocrit, Cr, and Hb having the largest effect.
Discussion
The importance of biomarkers is increasing in medicine. They have a relevant value in performing diagnosis and in predicting prognosis, treatment dose and in detecting efficacy and safety of drugs [32] and endeavour the sub-division individuals into different categories. In theory, a single biomarker may be ideal in clinical use to subdivide individuals but in the reality it hardly exists [33]. Here, using routinely biochemical and haematological tests, we evidenced that healthy, adult and non-smoking men and women (in follicular phase of menstrual cycle) have different biological landscapes. PCA analysis clearly shows the presence of male and female phenotypes that persist even after body weight normalization. Although, in line with previous results [7-9], the correction for body weight affects the statistical significance either qualitatively or quantitatively of the tests. The presence of male and female phenotypes when routine exams are performed is in line with previous results obtained with different parameters or different body fluids [34,35] such as the saliva of non-smokers. These findings suggest that PCA analysis is helpful into catch small sex-gender differences.
The changes induced by smoking, as opposed to those mediated by the correction for body weight, are able to abolish the male and female phenotype, indicating the great relevance of smoking in the generation of sex-gender differences.
Globally, these results indicate that only some sex-gender differences depend on the differences in body dimension highlighting the importance of demographic variables in generating sex-gender differences and similarities. Indeed, other investigations are needed to validate these findings and to elucidate their mechanistic and clinical significance. In addition, these findings rightly pose the question whether reference limits, for at least some values, should considered body weight normalization because sexual dimorphism is more evident when data were not corrected for body weight.
Indeed, the main and novel result is linked to the smoking effect that is able to compact male and female phenotypes, being present only a phenotype either before or after body weight normalization, indicating the importance of smoking in sex-gender studies as already suggested by WHO [12]. Therefore, these data suggest that sex-gender and smoking habit should be considered as independent variables in clinical trials in order to obtain rigorous scientific results when routine biomarkers were used. Further, these findings rightly pose the question whether reference values should consider the smoking habit. This is a very relevant point in consideration of the dimension of tobacco dependency over the world [10,11].
Conclusions
The present data suggest that the variables sex-gender and smoking habit should be included in the design and statistical analysis of clinical trials in order to reduce heterogeneity and to increase the adherence to real life. Randomised clinical trials performed with heterogeneous populations provide average effects of treatments that, although acceptable to regulatory authorities, may be insufficient for clinicians who focus their attention on the individual patient.
Acknowledgements
We thank Dr. Valentina Mirisola for her precious help in statistical analysis. This research was partially funded by a grant of INAIL “Bando BRIC 2016”.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Legato MJ. Gender in the genomic era Amsterdam. Boston: Elsevier Academic Press; 2017. Principles of gender-specific medicine. [Google Scholar]
- 2.Regitz-Zagrosek V. Sex and gender differences in pharmacology. Berlin: Springer-Verlag; 2012. [Google Scholar]
- 3.Franconi F, Campesi I. Sex impact on biomarkers, pharmacokinetics and pharmacodynamics. Curr Med Chem. 2017;24:2561–2575. doi: 10.2174/0929867323666161003124616. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz JB. The influence of sex on pharmacokinetics. Clin Pharmacokinet. 2003;42:107–121. doi: 10.2165/00003088-200342020-00001. [DOI] [PubMed] [Google Scholar]
- 5.Atkinson A, Abernethy D, Daniels C, Dedrick R, Markey S. Principles of clinical pharmacology. Academic Press; 2001. [Google Scholar]
- 6.Garcia-Lorda P, Bullo M, Balanza R, Salas-Salvado J. C-reactive protein, adiposity and cardiovascular risk factors in a Mediterranean population. Int J Obes (Lond) 2006;30:468–474. doi: 10.1038/sj.ijo.0803182. [DOI] [PubMed] [Google Scholar]
- 7.Campesi I, Occhioni S, Tonolo G, Cherchi S, Basili S, Carru C, Zinellu A, Franconi F. Ageing/menopausal status in healthy women and ageing in healthy men differently affect cardiometabolic parameters. Int J Med Sci. 2016;13:124–132. doi: 10.7150/ijms.14163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruoppolo M, Scolamiero E, Caterino M, Mirisola V, Franconi F, Campesi I. Female and male human babies have distinct blood metabolomic patterns. Mol Biosyst. 2015;11:2483–2492. doi: 10.1039/c5mb00297d. [DOI] [PubMed] [Google Scholar]
- 9.Karp NA, Mason J, Beaudet AL, Benjamini Y, Bower L, Braun RE, Brown SDM, Chesler EJ, Dickinson ME, Flenniken AM, Fuchs H, Angelis MH, Gao X, Guo S, Greenaway S, Heller R, Herault Y, Justice MJ, Kurbatova N, Lelliott CJ, Lloyd KCK, Mallon AM, Mank JE, Masuya H, McKerlie C, Meehan TF, Mott RF, Murray SA, Parkinson H, Ramirez-Solis R, Santos L, Seavitt JR, Smedley D, Sorg T, Speak AO, Steel KP, Svenson KL, Wakana S, West D, Wells S, Westerberg H, Yaacoby S, White JK. Prevalence of sexual dimorphism in mammalian phenotypic traits. Nat Commun. 2017;8:15475. doi: 10.1038/ncomms15475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pan A, Wang Y, Talaei M, Hu FB, Wu T. Relation of active, passive, and quitting smoking with incident type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015;3:958–967. doi: 10.1016/S2213-8587(15)00316-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooke JP. New insights into tobacco-induced vascular disease: clinical ramifications. Methodist Debakey Cardiovasc J. 2015;11:156–159. doi: 10.14797/mdcj-11-3-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organisation. Global Health Observatory (GHO) data. Prevalence of tobacco smoking. 2018. http://www.who.int/gho/tobacco/use/en/
- 13.Gregoraci G, van Lenthe FJ, Artnik B, Bopp M, Deboosere P, Kovacs K, Looman CWN, Martikainen P, Menvielle G, Peters F, Wojtyniak B, de Gelder R, Mackenbach JP. Contribution of smoking to socioeconomic inequalities in mortality: a study of 14 European countries, 1990-2004. Tob Control. 2017;26:260–268. doi: 10.1136/tobaccocontrol-2015-052766. [DOI] [PubMed] [Google Scholar]
- 14.Bobak M, Jha P, Nguyen S, Jarvis M. Poverty and smoking. In: Jha P, Chaloupka FJ, editors. Tobacco control in developing countries. Oxford: Oxford University Press; 2000. pp. 41–62. [Google Scholar]
- 15.Agabio R, Campesi I, Pisanu C, Gessa GL, Franconi F. Sex differences in substance use disorders: focus on side effects. Addict Biol. 2016;21:1030–1042. doi: 10.1111/adb.12395. [DOI] [PubMed] [Google Scholar]
- 16.Bauer T, Gohlmann S, Sinning M. Gender differences in smoking behavior. Health Econ. 2007;16:895–909. doi: 10.1002/hec.1259. [DOI] [PubMed] [Google Scholar]
- 17.Sieminska A, Jassem E. The many faces of tobacco use among women. Med Sci Monit. 2014;20:153–162. doi: 10.12659/MSM.889796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agabio R, Pisanu C, Gessa GL, Franconi F. Sex differences in alcohol use disorder. Curr Med Chem. 2017;24:2661–2670. doi: 10.2174/0929867323666161202092908. [DOI] [PubMed] [Google Scholar]
- 19.Fagiolino P, Vazquez M, Ibarra M, Magallanes L, Guevara N, Fotaki N. Sex- and smoke-related differences in gastrointestinal transit of cyclosporin A microemulsion capsules. Eur J Pharm Sci. 2014;63:140–146. doi: 10.1016/j.ejps.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 20.Ibarra M, Vazquez M, Fagiolino P. Sex effect on average bioequivalence. Clin Ther. 2017;39:23–33. doi: 10.1016/j.clinthera.2016.11.024. [DOI] [PubMed] [Google Scholar]
- 21.Zevin S, Benowitz NL. Drug interactions with tobacco smoking. An update. Clin Pharmacokinet. 1999;36:425–438. doi: 10.2165/00003088-199936060-00004. [DOI] [PubMed] [Google Scholar]
- 22.Oliveira P, Ribeiro J, Donato E, Madeira N. Smoking and antidepressants pharmacokinetics: a systematic review. Ann Gen Psychiatry. 2017;16:17. doi: 10.1186/s12991-017-0140-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goseva Z, Gjorcev A, Jovkovska Kaeva B, Janeva EJ, Angelovska I. Analysis of plasma concentrations of theophylline in smoking and nonsmoking patients with asthma. Open Access Maced J Med Sci. 2015;3:672–675. doi: 10.3889/oamjms.2015.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li H, Shi Q. Drugs and diseases interacting with cigarette smoking in US prescription drug labelling. Clin Pharmacokinet. 2015;54:493–501. doi: 10.1007/s40262-015-0246-6. [DOI] [PubMed] [Google Scholar]
- 25.Marino M, Masella R, Bulzomi P, Campesi I, Malorni W, Franconi F. Nutrition and human health from a sex-gender perspective. Mol Aspects Med. 2010;32:1–70. doi: 10.1016/j.mam.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 26.Fausto-Sterling A. Sex/Gender: biology in a social world. New York: Routledge; 2012. [Google Scholar]
- 27.Springer KW, Mager Stellman J, Jordan-Young RM. Beyond a catalogue of differences: a theoretical frame and good practice guidelines for researching sex/gender in human health. Soc Sci Med. 2012;74:1817–1824. doi: 10.1016/j.socscimed.2011.05.033. [DOI] [PubMed] [Google Scholar]
- 28.Campesi I, Carru C, Zinellu A, Occhioni S, Sanna M, Palermo M, Tonolo G, Mercuro G, Franconi F. Regular cigarette smoking influences the transsulfuration pathway, endothelial function, and inflammation biomarkers in a sex-gender specific manner in healthy young humans. Am J Transl Res. 2013;5:497–509. [PMC free article] [PubMed] [Google Scholar]
- 29.Ramsey JM, Cooper JD, Penninx BW, Bahn S. Variation in serum biomarkers with sex and female hormonal status: implications for clinical tests. Sci Rep. 2016;6:26947. doi: 10.1038/srep26947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campesi I, Sanna M, Zinellu A, Carru C, Rubattu L, Bulzomi P, Seghieri G, Tonolo G, Palermo M, Rosano G, Marino M, Franconi F. Oral contraceptives modify DNA methylation and monocyte-derived macrophage function. Biol Sex Differ. 2012;3:4. doi: 10.1186/2042-6410-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 32.Landeck L, Kneip C, Reischl J, Asadullah K. Biomarkers and personalized medicine: current status and further perspectives with special focus on dermatology. Exp Dermatol. 2016;25:333–339. doi: 10.1111/exd.12948. [DOI] [PubMed] [Google Scholar]
- 33.Zhou L, Beuerman R. The role of biomarker in personalized medicine: concept, technology and challenges. 2016. https://www.asiabiotech.com/15/1508/0014_0017.pdf.
- 34.Krumsiek J, Mittelstrass K, Do KT, Stuckler F, Ried J, Adamski J, Peters A, Illig T, Kronenberg F, Friedrich N, Nauck M, Pietzner M, Mook-Kanamori DO, Suhre K, Gieger C, Grallert H, Theis FJ, Kastenmuller G. Gender-specific pathway differences in the human serum metabolome. Metabolomics. 2015;11:1815–1833. doi: 10.1007/s11306-015-0829-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prodan A, Brand HS, Ligtenberg AJ, Imangaliyev S, Tsivtsivadze E, van der Weijden F, Crielaard W, Keijser BJ, Veerman EC. Interindividual variation, correlations, and sex-related differences in the salivary biochemistry of young healthy adults. Eur J Oral Sci. 2015;123:149–157. doi: 10.1111/eos.12182. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.