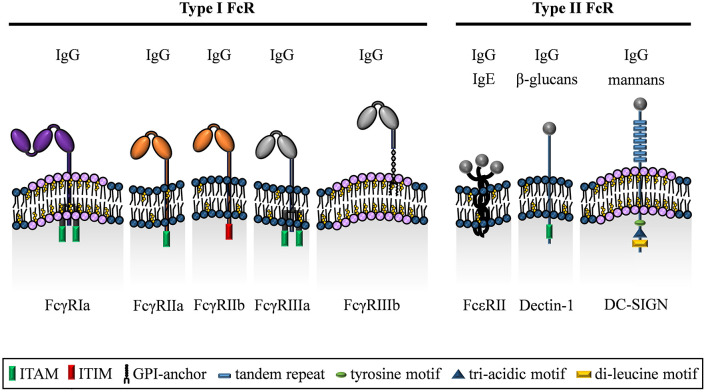
Figure 2.
The family of IgG binding type I and type II Fc receptors. The Fc receptors for IgG can be distinguished by their distinct binding mechanisms into either type I FcRs (classical FcγR) or type II FcRs (C-type lectin receptors). With respect to signal transduction, FcγRIa, FcγRIIa, FcγRIIIa, and Dectin-1 signal via ITAM domains in their cytoplasmic tail or on the associated FcεRγ chain. In contrast, the only inhibitory receptor FcγRIIb carries an ITIM domain in its cytoplasmic region. In addition, the GPI-linked FcγRIIIb lacks an intracellular signaling domain, but has a crucial role in binding of IgG immune complexes and enhances signaling upon cross-linking with other FcγRs. Besides, FcεRII has a short cytoplasmic region, whereas DC-SIGN exhibits a cytoplasmic domain including tyrosine, di-leucine and tri-acidic motifs. In addition to recognizing IgG, Dectin-1 and DC-SIGN are known to bind other ligands including defined sugar moieties.