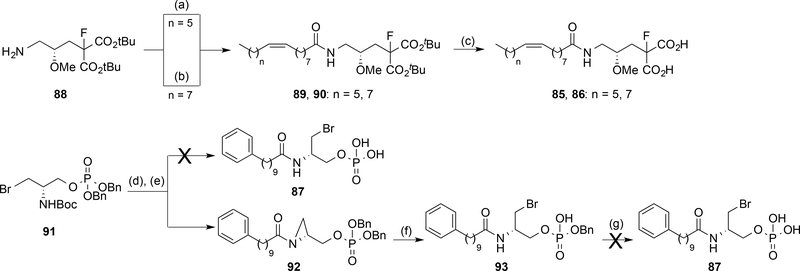
Scheme 6. a Synthesis of amides 85–87.
a Reagents and conditions: (a) palmitoleic acid, EDC, HOBt, DMF, rt, 18 h, 37%; (b) oleoyl chloride, Et3N, DCM, rt, 18 h, 46%; (c) TFA, DCM, rt, 18 h, 69–99%; (d) TFA, DCM, rt, 16 h, 99%; (e) N-hydroxysuccinimidyl ester of 53, Et3N, DCM, rt, 12 h, 48%; (f) MgBr2·OEt2, Et2O, rt, 4 h, 99%; (g) H2, 10% Pd/C, EtOH, rt.