Figure 3. Phase 2 trial design, patient disposition and responder analyses.
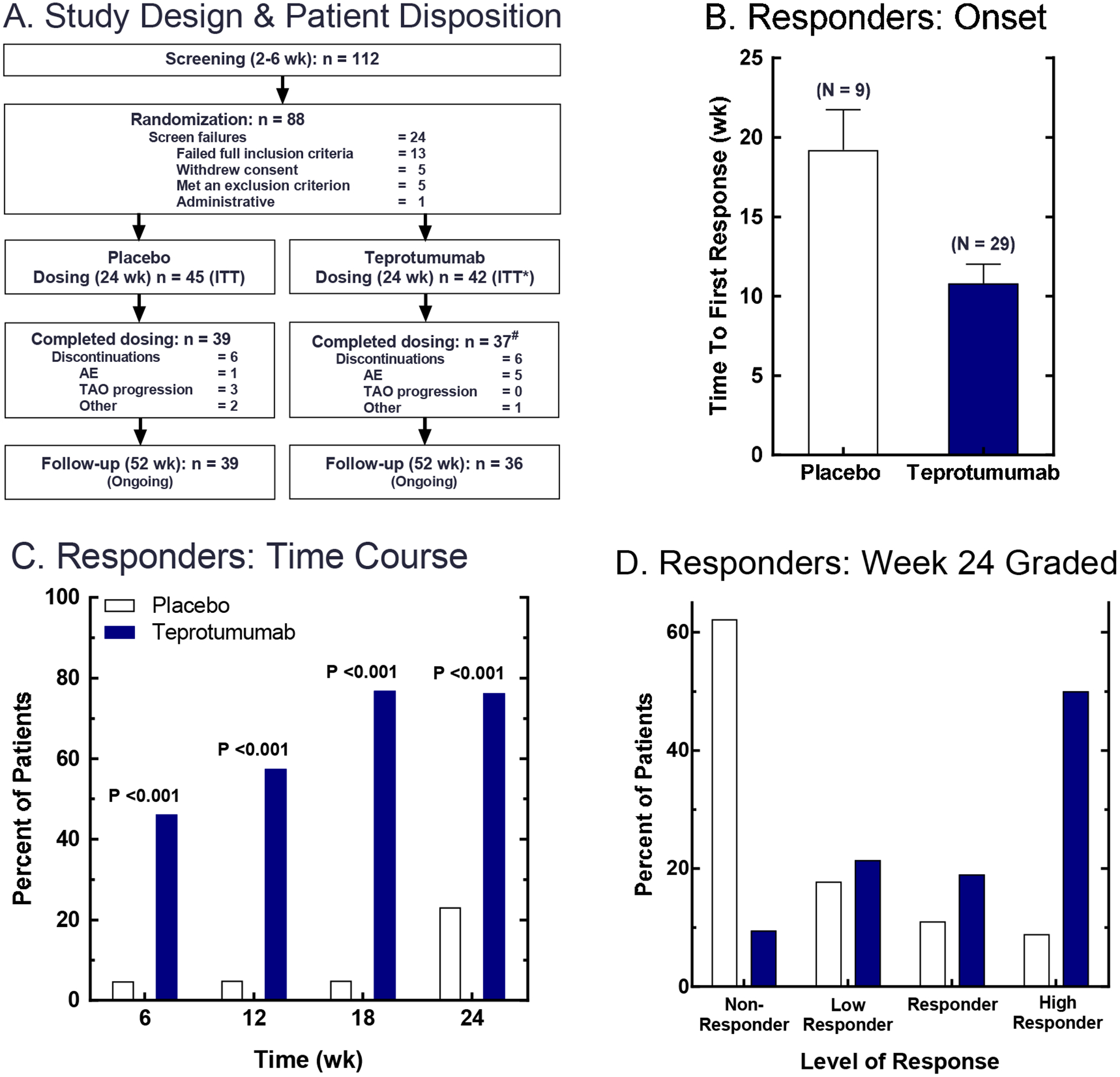
(Panel A) Patients who met primary inclusion criteria for disease onset ≤ 9 months and clinical activity score ≥ 4 were entered into the screening phase of the study. At the baseline visit, patients meeting all inclusion and exclusion criteria were randomized to receive teprotumumab or placebo. Teprotumumab was administered as 8 IV infusions, given at 3 week intervals over 24 weeks. Placebo was IV saline. (Panels B-D) Primary endpoint was a logistic regression on responder status at Week 24. Responders were defined as patients who had reduction in proptosis of ≥2 mm AND reduction in clinical activity score of ≥2 in the study eye. (Panel B) The time to first response (i.e. for meeting responder criteria); data are expressed as mean ± SE. (Panel C) Time course for patients meeting responder criteria. (Panel D) Grading of Week 24 responders; p <0.001 obtained from a logistic regression model. From N. Engl. J. Med, Smith T.J., Kahaly GJ, Ezra DG, Fleming JC, Dailey RA, et al, Teprotumumab for Thyroid-Associated Ophthalmopathy, 376:1748–61. Copyright © (2017) Massachusetts Medical Society. Reprinted with permission.
