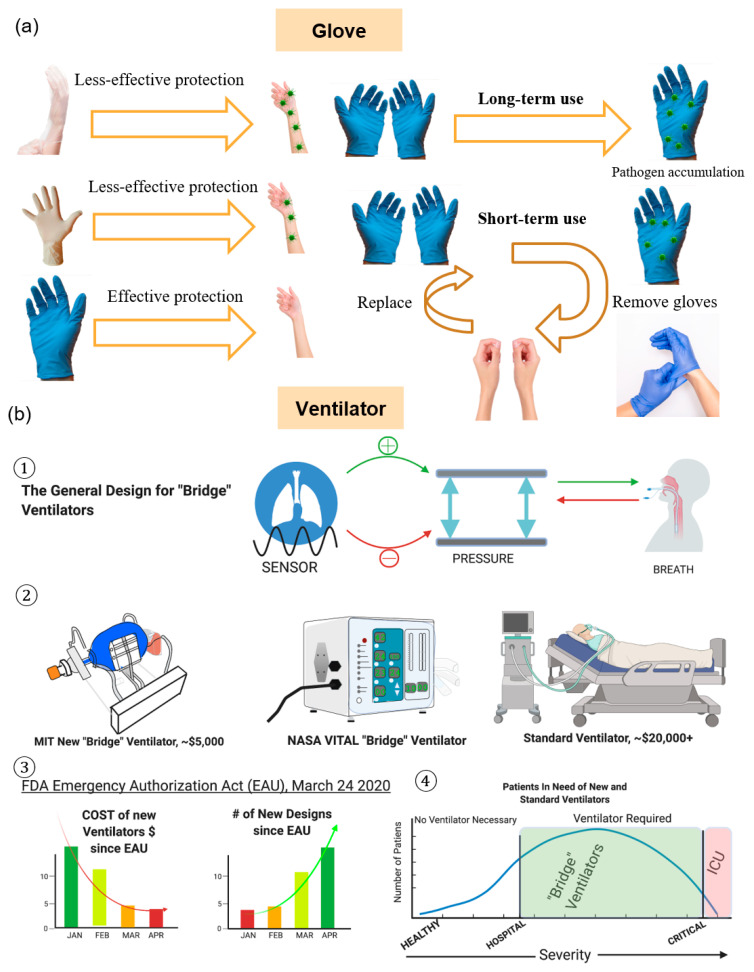
Figure 4.
COVID-19 control by gloves and ventilators. (a) Gloves must be changed regularly and properly disposed of in biosafe disposal units. Not all gloves block the penetration of the virus. Thicker gloves offer a thicker barrier and better defense against COVID-19 droplets; (b) (1) The general design for new ventilators includes a breath sensor that senses the timing and size of breaths, a pressure pump that pumps air into and out of the lungs, and a tracheal tube that pumps air directly into the lungs; (2) Depiction of the Massachusetts Institute of Technology (MIT) New “Bridge” ventilator which uses a mechanical pump and a bag-valve-mask to administer air (left). The National Aeronautics and Space Administration (NASA) VITAL uses sensors and a mechanical pump to deliver safe breaths. Their design is licensable to accepted manufacturers (middle). A standard intensive care unit (ICU) ventilator is fully equipped with vitals monitoring, air delivery, and a computer that measures vitals and pressure for safe breathing. The safest option for patients in critical condition but are more expensive to produce are presented (right); (3) The Food and Drug Administration (FDA)’s Emergency Use Authorization (EUA) for mechanical ventilators helped drastically reduce the cost of new devices and increased the number of new device designs; (4) Newly developed ventilators are termed “bridge” ventilators and are intended for an intermediate use between hospitalized patients and critically ill patients. “Bridge” ventilators are used when standard ICU ventilators are not available, and standard ICU ventilators are still the safest option for patients in critical condition.