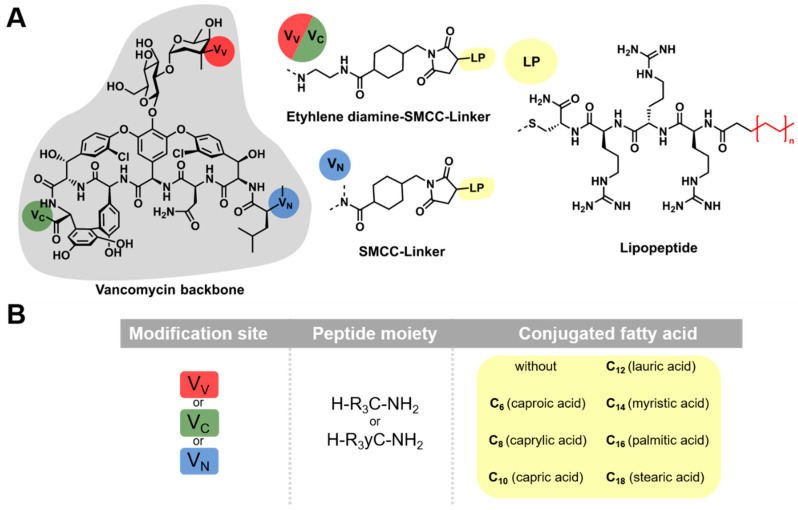
Figure 1.
Schematic illustration of vancomycin-lipopeptide conjugates. (A) Three different modification sites of vancomycin were addressed. The primary amine position VV (red) and the carboxylic acid position VC (green) were extended by a combination of ethylene diamine and the heterobifunctional crosslinker succinimidyl 4-(N-maleimidomethyl)cyclohexane-1-carboxylate (SMCC). The secondary amine position VN (blue) was extended with SMCC only. The maleimide function of SMCC enables the coupling of cysteine-containing lipopeptides (LP, yellow) via thiol maleimide Michael addition. For this coupling, peptide sequences containing cysteine (C) and tri-arginine (R3), combined with saturated fatty acids of varying chain lengths, were used. (B) Nomenclature of the generated conjugates. Conjugates are always designated in the described order: modification site (VV, VC, and VN)—peptide moiety (one-letter notation)—conjugated fatty acid.