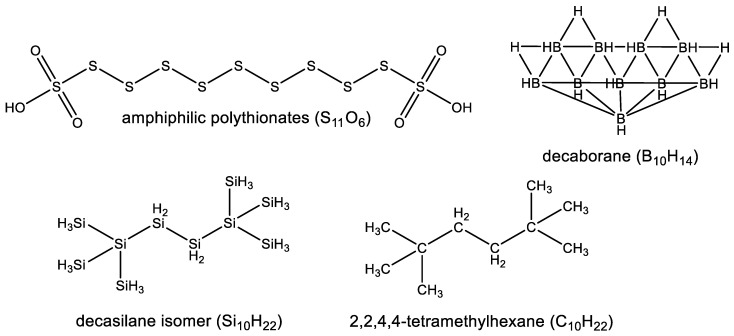
Figure 2.
Comparison of four (sulfur, boron, silicon and carbon) scaffolding elements and their pros and cons as main building blocks of biochemistry. Sulfur (e.g., amphiphilic polythionates [11]) forms chains with itself, and in an alternation with carbon, nitrogen, or oxygen (heteroatoms), but has a very limited branched structure, which severely limits the diversity of possible shapes in sulfur-based molecules. Boron (e.g., decaborane [12]) forms chains with itself, and in an alternation with carbon, nitrogen, or oxygen (heteroatoms), but forms clusters of atoms rather than smaller isolated molecules (a problem which is opposite to sulfur). Carbon (e.g., decane isomer) and silicon (e.g., decasilane isomer [15]), on the other hand, form chains with themselves, form chains in alternation with various heteroatoms, and can form diverse linear or branched structures. Out of the possible alternative scaffolding elements, silicon appears to be the most promising choice as a substituent for carbon in biochemistry.