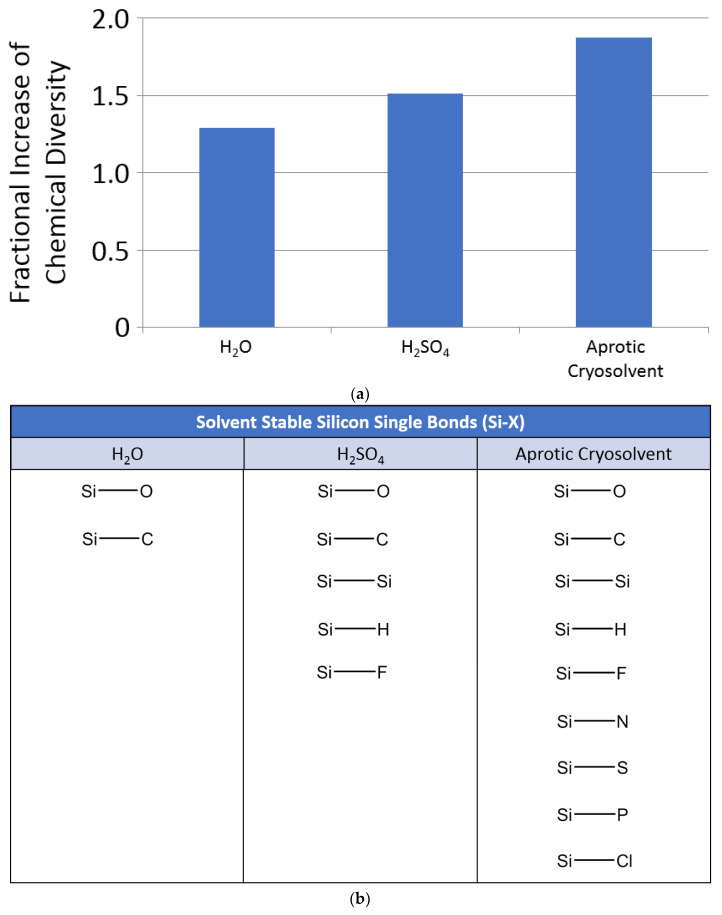
Figure 4.
Theoretical chemical diversity of silicon chemistry. (a) Relative size of the chemical space with silicon included in SPONCH + F + Cl chemistry, compared to the chemical space without silicon. Y axis: size of the chemical space of chemical structures with up to six non-hydrogen atoms predicted to be stable in respective solvents generated by including silicon chemistry. The relative size of the chemical space is calculated as S/N, where S is the number of molecules in the chemical space with silicon and N is the number without silicon. See main text and Appendix E.1 for details. (b) Silicon single bonds generally stable in H2O, H2SO4 and aprotic cryosolvent (a qualitative assessment, based on the chemical reactivity of silicon compounds discussed in detail in Section 4). Very few Si-containing chemical bonds are stable in water. Note that, while some Si–O bonds are generally stable to hydrolysis in water (e.g., in the context of C–Si bonds, like C–Si–OH), many Si–O-bond-containing functional groups are not (e.g., O–Si–O–C), further limiting the scope of Si chemistry available for life in water. Sulfuric acid can support larger number of Si-containing bonds, and, as a consequence, more diverse silicon chemistry. Virtually all chemistry is stable in aprotic cryosolvents.