Abstract
The etiology of schizophrenia is still unknown, and the MTHFR gene has been shown to be associated with SCZ. Previous studies have shown that patients with schizophrenia exhibit sex differences in symptoms and cognitive function. However, no study has been conducted to investigate the sex difference in the association between C677T polymorphism and symptoms and cognitive impairment in Chinese patients with schizophrenia. The C677T polymorphism was genotyped in 957 patients with schizophrenia and 576 controls. Patients were also rated on the Positive and Negative Syndrome Scale (PANSS) and the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS). The results showed that there were significant differences in MTHFR C677T genotype and allele distributions between male patients and male controls (both p<0.05), while there was no significant difference between female patients and female controls (both p>0.05). Further analysis showed that there were significant sex differences in the association between C677T genotype and negative symptoms, immediate memory or attention index score in schizophrenia (p<0.05). This study suggests that the complex interactive effect between MTHFR C677T polymorphism and sex plays an important role in some clinical characteristics of patients with schizophrenia.
Keywords: schizophrenia, symptoms, cognition, MTHFR, polymorphism
INTRODUCTION
Patients with schizophrenia (SCZ) exhibit cognitive impairments in several domains throughout the disease process [1, 2]. Cognitive impairment is recognized to be one of the core characteristics of SCZ, which includes attention, working memory, verbal learning and memory, and executive function [3]. However, the pathophysiological mechanisms of cognitive impairment in SCZ patients are still unclear, especially the biological pathological mechanism.
Accumulating studies have revealed that there are sex differences in the features of SCZ from the prevalence, symptoms, age at onset, illness course, to response to treatment [4–7]. For example, several reviews have demonstrated that female patients have an advantage over male patients in terms of age of onset, response to antipsychotic treatment, social function, and clinical symptoms, especially negative symptoms [4, 8–10]. However, female patients have more severe lipid metabolic dysfunction and more obesity than male patients [11]. More interestingly, some studies have investigated sex differences in cognitive impairment in patients with SCZ [4, 12, 13], indicating that men performed worse in attention, language and verbal memory and executive function [12, 14]. However, the results of sex differences in cognitive impairments and clinical symptoms were contradictory [15, 16]. Several studies have reported that there is no sex difference in cognitive impairment in patients with SCZ or even opposite results [16].
The methylenetetrahydrofolate reductase (MTHFR) gene is located on chromosome 1 and contains several common polymorphisms in its exons. The substitution (C to T) of nucleotide 677 in exon 4 results in amino acid substitution (Ala222Val) and decreased MTHFR activity [17]. Decreased MTHFR metabolism leads to hypomethylation of DNA, increases the concentrations of potentially toxic homocysteine, and causes abnormal levels of neurotransmitters [18–20].
Recently, the MTHFR C677T polymorphism has consistently reported to be related to SCZ, which has also been confirmed by several meta-analyses in Asian population [21–23]. Furthermore, a recent meta-analysis showed that the higher level of plasma total homocysteine was associated with the higher risk of SCZ [24]. Interestingly, the C677T polymorphism also played a critical role in the positive and negative symptoms of SCZ. For example, Roffman et al. found that SCZ patients with the TT genotype exhibited greater deficits in the verbal fluency test and more difficulties in the Wisconsin Card Sorting Test, but not in California Verbal Learning Test performance [25, 26]. In particular, a study in the Chinese population revealed that the TT genotype affected gray matter density and impaired memory in SCZ patients [27]. Taken together, these studies indicate that the C677T polymorphism may be involved in the psychopathology of SCZ patients.
Considering the significant sex differences in SCZ patients, the pathogenic role of MTHFR gene and cognitive impairment of SCZ, it is interesting to investigate whether there was a sex difference between the C667T polymorphism and cognitive impairment and clinical symptoms of SCZ patients. In particular, Wan et al. reported that male-specific effects of MTHFR polymorphism on the symptoms in chronic SZ patients [28]. However, the sample size is small and only one cognitive function domain was measured in that study. Based on previous literature, we hypothesized that when the patients were stratified by sex, the TT genotype of C677T was differently associated with symptoms and cognitive impairment in SCZ. Therefore, the purpose of the present study was to examine whether the interactive effects between C677T and sex would affect the clinical manifestations of SCZ patients.
RESULTS
Subject characteristics
The demographic and clinical characteristics were depicted in Table 1. There were significant differences in sex, age, smoking status and BMI between patients and healthy controls (all p< 0.01), which were controlled as confounding factors in the following analyses.
Table 1. Demographic characteristics, clinical data and MTHFR C677T genotypes in schizophrenia vs healthy controls.
| Variable | SCZ (n=957) | Controls (n=576) | F or χ2 (p) |
| Sex (male/female) | 783/174 | 263/313 | 216.8 (< 0.001) |
| Age (ys) | 47.8 ± 10.2 | 45.8 ± 12.8 | 11.7 (<0 .001) |
| Education (ys) | 9.3 ± 6.4 | 8.7 ± 3.2 | 1.5 (0.09) |
| Smokers/Nonsmokers | 627/318 | 218/358 | 117.7(<0 .001) |
| BMI (kg/m2) | 24.5 ± 3.9 | 25.1 ± 3.9 | 2.7 (<0 .01) |
| Drinking | 727/137 | 455/121 | 6.2(0.013) |
| Age at onset, mean ± SD, ys | 23.5 ± 5.7 | ||
| Duration of illness, mean ± SD, ys | 24.3 ± 10.0 | ||
| Antipsychotic dose (mg/d) | 455.2 ± 418.4 |
Notes: SCZ schizophrenia; ys years BMI body mass index.
Sex difference in the associations between C677T polymorphism and clinical symptoms in patients
The distribution of MTHFR C677T genotypes were in HWE in both patients and controls (both p>0.05). After adjusting for sex, smoking, body mass index (BMI) and age, there was no significant difference in the frequencies of C677T allele and genotype between SCZ patients and healthy controls (both p>0.05). After further analysis by sex grouping, there were significant differences in the frequencies of C677T genotype and allele between male patients and male controls (χ2 =7.6, p=0.023; χ2 =5.9, p=0.019, respectively, Table 2), but there was no significant difference between female patients and female controls (all p>0.05). Compared with the male control group, the prevalence of C677T-TT homozygous genotype was slightly higher in male patients (40.5% vs 28.9%). There was no significant difference in females (all p>0.05). Moreover, compared with C677T-CC heterozygous males, homozygous C677T-TT males were approximate 1.5-fold more likely to have SCZ (OR=1.51, 95% CI=1.0-2.3, p=0.042).
Table 2. MTHFR C677T allele and genotype frequencies in male and female of schizophrenia and healthy controls.
| Variable | SCZ (n=957) | Controls (n=576) | F or χ2 (p) |
| C677T genotype | 2.0(0.36) | ||
| CC (%) | 298(38.9) | 145(34.8) | |
| CT (%) | 344(45.0) | 202(48.4) | |
| TT (%) | 123(16.1) | 70(16.8) | |
| Male (CC/CT/TT) | 7.6(0.023) | ||
| CC (%) | 101(15.8) | 31(18.7) | |
| CT (%) | 280(43.7) | 87(52.4) | |
| TT (%) | 260(40.5) | 48(28.9) | |
| Female (CC/CT/TT) | 2.3(0.32) | ||
| CC (%) | 22(17.7) | 38(15.3) | |
| CT (%) | 64(51.6) | 115(46.2) | |
| TT (%) | 38(30.6) | 96(38.5) | |
| C677T allele (C/T) | 590/940 | 342/492 | 0.08(0.83) |
| Male (C/T) | 482/800 | 149/183 | 5.9(0.015) |
| Female (C/T) | 108/140 | 191/307 | 1.9(0.17) |
There were significant differences in symptoms between male and female patients. Male patients had less severe positive symptoms, negative symptoms, general symptoms and PANSS total score than female patients (all p<0.05).
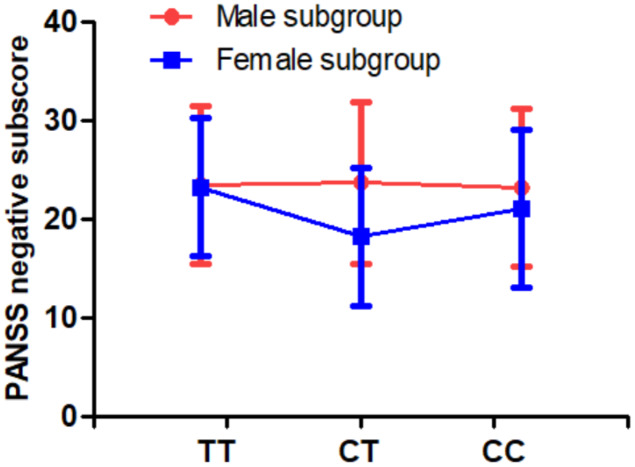
In addition, we found that there were differences in PANSS negative symptoms between MTHFR C677T genotypes (p=0.031). Multivariate analysis of covariance also revealed a significant sex difference in the association between C677T genotype and PANSS negative symptoms (p=0.015, Table 3 and Figure 1). Further analysis found that female patients with CT genotype had fewer severe negative symptoms than male patients with CT genotype.
Table 3. Sex differences of clinical symptoms and cognitive functions in the patients with schizophrenia and healthy controls.
| Male | Female | Sex p | Genotype p | Sex×gene p | |||||
| TT | CT | CC | TT | CT | CC | ||||
| Patients with SCZ | |||||||||
| Cognitive functions | |||||||||
| Immediate memory | 56.3±14.5 | 58.0±15.7 | 58.4±16.8 | 64.0±13.9 | 68.8±22.9 | 58.7±13.9 | 0.017 | 0.06 | 0.05 |
| Visuospatial/constructional | 75.3±17.9 | 76.5±18.2 | 78.0±20.1 | 81.0±18.1 | 83.9±19.1 | 84.9±22.3 | 0.010 | 0.58 | 0.95 |
| Language | 81.0±14.6 | 80.7±15.4 | 80.3±16.6 | 83.3±16.3 | 88.9±15.8 | 80.7±15.0 | 0.084 | 0.23 | 0.24 |
| Attention | 67.5±17.3 | 70.0±16.7 | 71.1±18.6 | 83.0±15.2 | 78.3±17.1 | 71.5±17.8 | 0.001 | 0.46 | 0.05 |
| Delayed memory | 62.3±17.7 | 67.2±19.1 | 65.0±19.2 | 76.4±20.3 | 79.3±23.0 | 67.9±17.4 | <0.001 | 0.04 | 0.26 |
| Total score | 61.7±13.4 | 63.5±14.6 | 64.4±14.8 | 71.8±14.4 | 75.0±19.0 | 65.9±14.9 | <0.001 | 0.22 | 0.14 |
| Clinical symptoms | |||||||||
| Positive symptom | 11.2±4.5 | 11.1±4.6 | 11.5±4.4 | 13.2±5.6 | 14.9±6.6 | 14.0±6.7 | <0.001 | 0.37 | 0.24 |
| Negative symptom | 23.5±8.0 | 23.7±8.2 | 23.2±8.0 | 23.2±7.3 | 18.2±9.0 | 21.0±8.1 | 0.002 | 0.031 | 0.015 |
| General psychopathology | 25.0±5.5 | 24.7±5.6 | 25.5±6.2 | 28.8±6.3 | 27.4±5.6 | 28.8±8.5 | <0.001 | 0.27 | 0.72 |
| PANSS total score | 59.7±14.5 | 59.5±14.6 | 60.2±14.7 | 65.2±14.6 | 60.5±14.8 | 63.7±19.4 | 0.04 | 0.29 | 0.39 |
| Healthy controls | |||||||||
| Cognitive functions | |||||||||
| Immediate memory | 74.9±17.0 | 76.2±15.3 | 74.4±15.7 | 73.3±17.3 | 77.8±18.3 | 74.6±20.0 | 0.97 | 0.32 | 0.74 |
| Visuospatial/constructional | 80.4±15.1 | 82.1±16.6 | 80.3±14.7 | 77.1±14.9 | 79.7±16.9 | 77.4±12.2 | 0.11 | 0.44 | 0.97 |
| Language | 95.2±9.1 | 94.0±12.0 | 97.5±13.4 | 93.0±14.1 | 93.3±13.3 | 92.3±16.5 | 0.08 | 0.80 | 0.48 |
| Attention | 87.5±21.0 | 90.5±16.3 | 87.9±20.5 | 83.5±22.0 | 85.9±22.4 | 92.9±15.6 | 0.61 | 0.27 | 0.24 |
| Delayed memory | 89.2±12.5 | 84.7±14.4 | 88.7±15.5 | 85.0±16.1 | 87.1±16.3 | 85.6±14.5 | 0.31 | 0.79 | 0.12 |
| Total score | 80.6±13.6 | 81.1±13.1 | 81.9±15.2 | 77.5±15.9 | 80.2±16.6 | 80.0±15.1 | 0.26 | 0.60 | 0.83 |
Figure 1.
There was a significant interaction of sex and MTHFR C677T genotype on PANSS negative symptoms (p=0.015).
Sex difference in the associations between MTHFR C677T polymorphism and cognitive functions both in patients and controls
Female patients performed better than male patients in terms of delayed memory, attention, visuospatial/constructional, immediate memory and RBANS total score (all p<0.05). Moreover, we found that the C677T genotype was related to delayed memory index (F=3.4, p=0.036, Table 3) in patients, but not in controls. There was no significant difference in cognitive function between patients using typical and atypical antipsychotics.
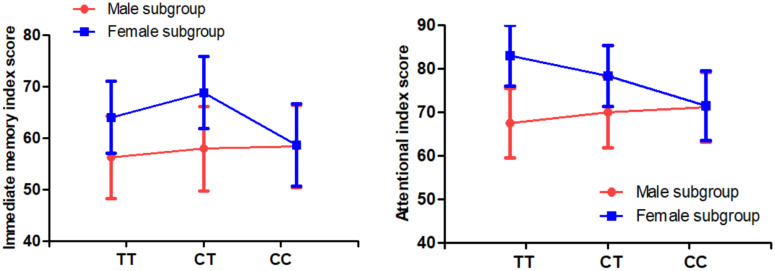
Multivariate analysis of covariance also revealed that there was a trend toward a significant sex difference in the relationship between C677T genotype and immediate memory and attention in patients (all p=0.05), but not in controls (all p>0.05). Further analysis found that female patients with TT and CT genotypes performed better on immediate memory and attention than male patients with TT and CT genotypes, while female patients with CC genotype had similar cognitive function as male patients with CC genotype (Table 3 and Figure 2).
Figure 2.
There were significant interactions of sex and MTHFR C677T genotypes on immediate memory and attention index score in the patients with SCZ (all p<0.05).
DISCUSSION
There were three major findings in the current study. First, the C677T polymorphism was susceptible to SCZ in male patients and was associated with PANSS negative symptoms and RBANS cognitive impairment. Second, except for RBANS language index, the RBANS total score and all 5 index scores in male patients were lower than those in female patients. Third, there were significant sex differences in the association between C677T polymorphism and negative symptoms and cognitive impairment (including immediate memory and attention), suggesting that the multifactorial etiology involving MTHFR gene and sex may be involved in the pathophysiology of SCZ patients.
Some previous studies have examined the possible impact of MTHFR gene on susceptibility to SCZ, but the results vary in different populations. Our findings provide more evidence that MTHFR C677T polymorphism is a risk factor for Chinese male SCZ patients. Then, we found that this polymorphism was correlated to delayed memory in SCZ patients, which was in accordance to previous studies [26, 29–31]. For example, Kontis et al. found that there was a significant interactive effect between MTHFR and COMT genes on spatial working memory in chronic SCZ patients and healthy controls [29]. Our results were comparable to those found by Kontis, supporting that the memory loss in SCZ patients was influenced by the MTHFR gene. However, we did not find a relationship between C677T and cognition in controls, which was inconsistent with the finding of Kontis et al. [29]. In this study, male patients performed worse than female patients on most cognitive tasks measured by RBANS, which was in line with most previous studies [32–34]. A possible explanation for the better cognitive function of female than male subjects may be associated with the influence of sex hormones on cognitive function. Studies have shown that testosterone and estrogen may play a critical role in cognition through the effects of dopamine and serotonin on certain brain regions, as reviewed in previous reports [35, 36]. In particular, the findings of preclinical studies demonstrated that estrogen can reduce the concentrations of dopamine and the number of dopamine receptors in the hippocampus [37–39]. Moreover, numerous studies have revealed that the average age of onset of symptoms in female SCZ patients was later than that in male patients [40, 41]. The neurodevelopmental hypothesis postulated that SCZ patients with an earlier onset age have more severe central nervous system damage and poorer cognitive function than those with later age of onset [14, 42, 43]. Therefore, estrogen may be the reason why female patients perform better in several domains of cognitive functions. Also, in this study, we found that men had more severe negative symptoms than women, which may be the reason for the decline in cognitive function. However, the exact mechanisms of male disadvantage in multiple cognitive domains warrants further preclinical and clinical investigations.
Another finding of this study was that an interplay effect of sex and C677T polymorphism on negative symptoms was found in patients. Female patients with CT genotype had fewer negative symptoms than female patients with CC and TT genotypes and male patients, suggesting that C677T genotype had heterozygous effect on negative symptom. Our results were partially consistent to previous studies in SCZ patients [25, 28]. Interestingly, we also found a relationship between C677T polymorphism, sex and cognitive function or negative symptoms in patients, but not in healthy controls. This is consistent with a previous study by Roffman et al. [26], which found that the MTHFR gene impaired cognitive function, taking into account the mediating effect of negative symptoms. We speculate that the interactive effect between CT genotype × sex and cognitive impairment may be associated with altered MTHFR enzyme activity. Studies have shown that MTHFR gene was correlated with MTHFR enzyme activity, and individuals with CT and TT genotypes have only 67% and 25% of enzyme activity of their CC genotype counterparts, respectively [17].
The lower enzyme activity in male patients may elevate the concentration of homocysteine in patients with insufficient dietary folate intake [44, 45]. A large number of studies have also demonstrated that elevated total homocysteine levels in blood were one of the most important risk factors for cognitive impairment in SCZ, and lowering the homocysteine concentration in patients with hyperhomocysteinemia may predict the improvement of cognitive function [46, 47]. However, it is worth mentioning that the reason why this C677T polymorphism was associated with negative symptoms and cognition may be due to the enzymatic effects of genotypes, which is only our speculation. According to previous studies on MTHFR enzyme activity, patients with homozygous wildtype CC should have fewer negative symptoms and better cognitive function than patients with homozygous (TT) and heterozygous (CT) genotypes. However, the fact is that the negative symptoms of patients with TT and CC genotype were almost equal, and the difference is not significantly (Table 3). In female patients, although there were significant differences in cognitive function between TT and CC genotype subjects, CC subjects were worse than TT subjects, which exceed our expectations. Taken together, this interpretation of the association between genotypes and negative symptoms or cognition due to the enzymatic effects of genotypes is quite speculative and may only provide part of the reason. The mechanism connecting MTHFR C677T gene polymorphism and SCZ is still unknown. In order to fully understand the relationship between MTHFR genetic variants and SCZ, it will be necessary to further combine multiple MTHFR genetic polymorphisms in larger samples for haplotype analysis.
There are several limitations that should be noted. First, the influence of MTHFR TT genotype on MTHFR enzyme activities was partially weakened by taking more folate and cobalamin [48]. However, in the present study, plasma folate and homocysteine levels of individuals were not measured. Also, we did not collect relevant information, nor did we know whether the participants recruited in this study were folic-acid-fortified people. Second, many studies have found that other factors may exacerbate folate deficiency, for example, older age, obesity and alcoholism [49, 50], which, unfortunately were not collected in this study. Third, there were more male than female subjects in SCZ patients, which may have led to bias in statistical analysis due to the imbalance of the number of subjects in each sex category in this study. Fourth, because atypical and typical antipsychotics have different effects on cognitive function, and patients were treated with different types of antipsychotics in this study, we could not exclude the effects of antipsychotic drugs.
In summary, in the pilot study, we showed the relationship between the MTHFR C677T polymorphism and SCZ in male patients, and this polymorphism was significantly associated with symptoms. Moreover, the C677T polymorphism was correlated with the delayed memory of the patients. Female patients had fewer symptoms and better cognition than male patients. We observed sex difference in the relationship between C677T polymorphism and negative symptoms, immediate memory and attention index scores. However, caution is needed in interpreting the results. Considering the small sample size and the different sex composition, the significant association between MTHFR C677T polymorphism and symptoms or cognition may be due to statistical bias. The sex difference in the relationship between MTHFR C677T polymorphism and clinical characteristics needs to be further studied in a larger sample of more female patients.
MATERIALS AND METHODS
Subjects
The participants were recruited from January 10, 2010 to December 25, 2013. A total of 957 SCZ patients were enrolled from Beijing Huilongguan Hospital and HeBei Province Veteran Psychiatric Hospital located in northern China. The inclusion criteria were as follows: (1) between the ages of 20 to 75 years; (2) Han Chinese; (3) diagnosed as SCZ confirmed by the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV); (4) at least 60 months of illness; (5) taking a stable dose of antipsychotics for at least six months; (6) providing written informed consent. Antipsychotic drugs mainly consisted of drug monotherapy, including risperidone (n =210), clozapine (n= 430), sulpiride (n =49), chlorpromazine (n =69), quetiapine (n = 42), perphenazine (n = 45), haloperidol (n = 34), aripirazole (n = 29), and others (n = 49). The average daily dose of antipsychotics (in term of chlorpromazine equivalent) was 438 ± 407 mg/day.
During the same period, 576 healthy controls were randomly recruited through advertisements in the local community. Six psychiatrists screened and excluded potential controls with Axis I disorders at present or in their lifetime after a structured clinical interview. All control subjects were Han Chinese.
All subjects underwent extensive physical examinations and laboratory tests when they were recruited. Any participants with abnormal health were excluded. All subjects signed the written informed consent, which was approved by the Institutional Review Board of Beijing HuiLongGuan hospital.
Assessments
The 4 clinical psychiatrists evaluated the psychiatric symptoms of SCZ by the Positive and Negative Syndrome Scale (PANSS) on the same day of blood sampling. In this study, these psychiatrists were trained to assess PANSS. After training, they maintained a correlation coefficient ≥0.8 for the reliability of the repeated evaluation of PANSS total score. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS, Form A) was used to assess the cognitive function of all participants. RBANS consists of five index scores and a total score. The five indexes include immediate memory, attention, visuospatial/constructional, language and delayed memory. Our group has translated RBANS into Chinese and established the clinical validity and test-retest reliability of the Chinese version among SCZ patients and controls.
C677T polymorphism analysis
C677T polymorphism was genotyped by MALDI-TOF MS platform (Sequenom, CA, USA), following the standard procedure as described in sequenom genotyping protocol, which is considered efficient and accurate [51]. The MassARRAY system is an extensible platform that provides a set of applications for quantitative and qualitative genomic analysis. Primers for PCR and single base extension were designed by using the Sequenom MassARRAY Assay Designer software v3. The primers of C677T were: sense: 5′-ACGTTGGATGCTTGAAGGAGAAGGTGTCTG-3′, antisense: 5′- ACGTTGGATGCTTCACAAAGCGGAAGAATG-3′, and extent probe: AAGGTGTCTGCGGGAG.
The sample genotyping success rate in this study averaged 98.9%. 5% of all DNA samples were repeated, showing 99.5% reproducibility of SNP results.
Statistical analysis
Hardy-Weinberg equilibrium (HWE) was examined using χ2 test. The differences in the allele and genotype frequencies of C677T polymorphism between the two groups were analyzed by using χ2 test. Then, analysis of variance (ANOVA) was performed to analyze the association between sex or MTHFR genotype and cognitive function or symptoms. Confounding factors included age, smoking, BMI, education, course of illness, antipsychotic type and dose. We paid more attention to the interaction term (sex × MTHFR genotype) because there may be any potential differences between different groups.
We used SPSS version 15.0 to perform all statistical analyses, with two-tailed p values of less than 0.05. Bonferroni correction was used for each analysis to correct multiple tests. The power of the sample was calculated by using Quanto software, with the relative risk and known risk allele frequencies under log additive, recessive and dominant models, respectively.
Footnotes
CONFLICTS OF INTEREST: No conflict of interest was disclosed for each author.
FUNDING: This study was supported by the grants from the National Natural Science Foundation of China (81371477, 81000509), Beijing Natural Science Foundation (7194270) and Beijing Hospitals Authority Youth Programme (QML20190307). These sources had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
REFERENCES
- 1.Schaefer J, Giangrande E, Weinberger DR, Dickinson D. The global cognitive impairment in schizophrenia: consistent over decades and around the world. Schizophr Res. 2013; 150:42–50. 10.1016/j.schres.2013.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bora E, Özerdem A. Meta-analysis of longitudinal studies of cognition in bipolar disorder: comparison with healthy controls and schizophrenia. Psychol Med. 2017; 47:2753–66. 10.1017/S0033291717001490 [DOI] [PubMed] [Google Scholar]
- 3.Gold JM, Robinson B, Leonard CJ, Hahn B, Chen S, McMahon RP, Luck SJ. Selective Attention, Working Memory, and Executive Function as Potential Independent Sources of Cognitive Dysfunction in Schizophrenia. Schizophr Bull. 2018; 44:1227–34. 10.1093/schbul/sbx155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mendrek A, Mancini-Marïe A. Sex/gender differences in the brain and cognition in schizophrenia. Neurosci Biobehav Rev. 2016; 67:57–78. 10.1016/j.neubiorev.2015.10.013 [DOI] [PubMed] [Google Scholar]
- 5.Abel KM, Drake R, Goldstein JM. Sex differences in schizophrenia. Int Rev Psychiatry. 2010; 22:417–28. 10.3109/09540261.2010.515205 [DOI] [PubMed] [Google Scholar]
- 6.Zhang XY, Chen DC, Xiu MH, Yang FD, Haile CN, Kosten TA, Kosten TR. Gender differences in never-medicated first-episode schizophrenia and medicated chronic schizophrenia patients. J Clin Psychiatry. 2012; 73:1025–33. 10.4088/JCP.11m07422 [DOI] [PubMed] [Google Scholar]
- 7.Talonen S, Väänänen J, Kaltiala-Heino R. Gender differences in first onset schizophrenia spectrum psychoses. Nord J Psychiatry. 2017; 71:131–38. 10.1080/08039488.2016.1245783 [DOI] [PubMed] [Google Scholar]
- 8.Seeman MV. Does gender influence outcome in schizophrenia? Psychiatr Q. 2019; 90:173–84. 10.1007/s11126-018-9619-y [DOI] [PubMed] [Google Scholar]
- 9.McGregor C, Riordan A, Thornton J. Estrogens and the cognitive symptoms of schizophrenia: Possible neuroprotective mechanisms. Front Neuroendocrinol. 2017; 47:19–33. 10.1016/j.yfrne.2017.06.003 [DOI] [PubMed] [Google Scholar]
- 10.Esterberg ML, Trotman HD, Holtzman C, Compton MT, Walker EF. The impact of a family history of psychosis on age-at-onset and positive and negative symptoms of schizophrenia: a meta-analysis. Schizophr Res. 2010; 120:121–30. 10.1016/j.schres.2010.01.011 [DOI] [PubMed] [Google Scholar]
- 11.Li Q, Chen D, Liu T, Walss-Bass C, de Quevedo JL, Soares JC, Zhao J, Zhang XY. Sex differences in body mass index and obesity in chinese patients with chronic schizophrenia. J Clin Psychopharmacol. 2016; 36:643–48. 10.1097/JCP.0000000000000594 [DOI] [PubMed] [Google Scholar]
- 12.Hamson DK, Roes MM, Galea LA. Sex hormones and cognition: neuroendocrine influences on memory and learning. Compr Physiol. 2016; 6:1295–337. 10.1002/cphy.c150031 [DOI] [PubMed] [Google Scholar]
- 13.Leger M, Neill JC. A systematic review comparing sex differences in cognitive function in schizophrenia and in rodent models for schizophrenia, implications for improved therapeutic strategies. Neurosci Biobehav Rev. 2016; 68:979–1000. 10.1016/j.neubiorev.2016.06.029 [DOI] [PubMed] [Google Scholar]
- 14.Goldstein JM, Seidman LJ, Goodman JM, Koren D, Lee H, Weintraub S, Tsuang MT. Are there sex differences in neuropsychological functions among patients with schizophrenia? Am J Psychiatry. 1998; 155:1358–64. 10.1176/ajp.155.10.1358 [DOI] [PubMed] [Google Scholar]
- 15.Navarra-Ventura G, Fernandez-Gonzalo S, Turon M, Pousa E, Palao D, Cardoner N, Jodar M. Gender differences in social cognition: a cross-sectional pilot study of recently diagnosed patients with schizophrenia and healthy subjects. Can J Psychiatry. 2018; 63:538–46. 10.1177/0706743717746661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ayesa-Arriola R, Rodriguez-Sanchez JM, Gomez-Ruiz E, Roiz-Santiáñez R, Reeves LL, Crespo-Facorro B. No sex differences in neuropsychological performance in first episode psychosis patients. Prog Neuropsychopharmacol Biol Psychiatry. 2014; 48:149–54. 10.1016/j.pnpbp.2013.09.009 [DOI] [PubMed] [Google Scholar]
- 17.Wan L, Li Y, Zhang Z, Sun Z, He Y, Li R. Methylenetetrahydrofolate reductase and psychiatric diseases. Transl Psychiatry. 2018; 8:242. 10.1038/s41398-018-0276-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lightfoot TJ, Johnston WT, Painter D, Simpson J, Roman E, Skibola CF, Smith MT, Allan JM, Taylor GM, and United Kingdom Childhood Cancer Study. Genetic variation in the folate metabolic pathway and risk of childhood leukemia. Blood. 2010; 115:3923–29. 10.1182/blood-2009-10-249722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burghardt KJ, Pilsner JR, Bly MJ, Ellingrod VL. DNA methylation in schizophrenia subjects: gender and MTHFR 677C/T genotype differences. Epigenomics. 2012; 4:261–68. 10.2217/epi.12.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bahous RH, Cosín-Tomás M, Deng L, Leclerc D, Malysheva O, Ho MK, Pallàs M, Kaliman P, Bedell BJ, Caudill MA, Rozen R. Early manifestations of brain aging in mice due to low dietary folate and mild MTHFR deficiency. Mol Neurobiol. 2019; 56:4175–91. 10.1007/s12035-018-1375-3 [DOI] [PubMed] [Google Scholar]
- 21.Yadav U, Kumar P, Gupta S, Rai V. Role of MTHFR C677T gene polymorphism in the susceptibility of schizophrenia: an updated meta-analysis. Asian J Psychiatr. 2016; 20:41–51. 10.1016/j.ajp.2016.02.002 [DOI] [PubMed] [Google Scholar]
- 22.Hu CY, Qian ZZ, Gong FF, Lu SS, Feng F, Wu YL, Yang HY, Sun YH. Methylenetetrahydrofolate reductase (MTHFR) polymorphism susceptibility to schizophrenia and bipolar disorder: an updated meta-analysis. J Neural Transm (Vienna). 2015; 122:307–20. 10.1007/s00702-014-1261-8 [DOI] [PubMed] [Google Scholar]
- 23.Zintzaras E. C677T and A1298C methylenetetrahydrofolate reductase gene polymorphisms in schizophrenia, bipolar disorder and depression: A meta-analysis of genetic association studies. Psychiatr Genet. 2006; 16:105–15. 10.1097/01.ypg.0000199444.77291.e2 [DOI] [PubMed] [Google Scholar]
- 24.Nishi A, Numata S, Tajima A, Kinoshita M, Kikuchi K, Shimodera S, Tomotake M, Ohi K, Hashimoto R, Imoto I, Takeda M, Ohmori T. Meta-analyses of blood homocysteine levels for gender and genetic association studies of the MTHFR C677T polymorphism in schizophrenia. Schizophr Bull. 2014; 40:1154–63. 10.1093/schbul/sbt154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roffman JL, Weiss AP, Purcell S, Caffalette CA, Freudenreich O, Henderson DC, Bottiglieri T, Wong DH, Halsted CH, Goff DC. Contribution of methylenetetrahydrofolate reductase (MTHFR) polymorphisms to negative symptoms in schizophrenia. Biol Psychiatry. 2008; 63:42–48. 10.1016/j.biopsych.2006.12.017 [DOI] [PubMed] [Google Scholar]
- 26.Roffman JL, Weiss AP, Deckersbach T, Freudenreich O, Henderson DC, Purcell S, Wong DH, Halsted CH, Goff DC. Effects of the methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism on executive function in schizophrenia. Schizophr Res. 2007; 92:181–88. 10.1016/j.schres.2007.01.003 [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Yan H, Tian L, Wang F, Lu T, Wang L, Yan J, Liu Q, Kang L, Ruan Y, Zhang D, Yue W. Association of MTHFR C677T polymorphism with schizophrenia and its effect on episodic memory and gray matter density in patients. Behav Brain Res. 2013; 243:146–52. 10.1016/j.bbr.2012.12.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wan L, Zhang G, Liu M, Wang C, Li Y, Li R. Sex-specific effects of methylenetetrahydrofolate reductase polymorphisms on schizophrenia with methylation changes. Compr Psychiatry. 2019; 94:152121. 10.1016/j.comppsych.2019.152121 [DOI] [PubMed] [Google Scholar]
- 29.Kontis D, Theochari E, Fryssira H, Kleisas S, Sofocleous C, Andreopoulou A, Kalogerakou S, Gazi A, Boniatsi L, Chaidemenos A, Tsaltas E. COMT and MTHFR polymorphisms interaction on cognition in schizophrenia: an exploratory study. Neurosci Lett. 2013; 537:17–22. 10.1016/j.neulet.2013.01.012 [DOI] [PubMed] [Google Scholar]
- 30.Roffman JL, Weiss AP, Deckersbach T, Freudenreich O, Henderson DC, Wong DH, Halsted CH, Goff DC. Interactive effects of COMT Val108/158Met and MTHFR C677T on executive function in schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2008; 147B:990–95. 10.1002/ajmg.b.30684 [DOI] [PubMed] [Google Scholar]
- 31.Wang W, Fan W, Shi B, Tong C, Wang X, Cai J, Zhang C. [Effect of MTHFR gene on the schizophrenia and its cognitive function]. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2017; 34:905–08. 10.3760/cma.j.issn.1003-9406.2017.06.028 [DOI] [PubMed] [Google Scholar]
- 32.Condray R, Yao JK. Cognition, dopamine and bioactive lipids in schizophrenia. Front Biosci (Schol Ed). 2011; 3:298–330. 10.2741/s153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang XY, Chen DC, Tan YL, Tan SP, Wang ZR, Yang FD, Xiu MH, Hui L, Lv MH, Zunta-Soares GB, Soares JC. Gender difference in association of cognition with BDNF in chronic schizophrenia. Psychoneuroendocrinology. 2014; 48:136–46. 10.1016/j.psyneuen.2014.06.004 [DOI] [PubMed] [Google Scholar]
- 34.Zhang BH, Han M, Zhang XY, Hui L, Jiang SR, Yang FD, Tan YL, Wang ZR, Li J, Huang XF. Gender differences in cognitive deficits in schizophrenia with and without diabetes. Compr Psychiatry. 2015; 63:1–9. 10.1016/j.comppsych.2015.07.003 [DOI] [PubMed] [Google Scholar]
- 35.Fink G, Sumner B, Rosie R, Wilson H, McQueen J. Androgen actions on central serotonin neurotransmission: relevance for mood, mental state and memory. Behav Brain Res. 1999; 105:53–68. 10.1016/s0166-4328(99)00082-0 [DOI] [PubMed] [Google Scholar]
- 36.Riecher-Rössler A, Häfner H. Gender aspects in schizophrenia: bridging the border between social and biological psychiatry. Acta Psychiatr Scand Suppl. 2000; 407:58–62. 10.1034/j.1600-0447.2000.00011.x [DOI] [PubMed] [Google Scholar]
- 37.Woolley CS, McEwen BS. Estradiol regulates hippocampal dendritic spine density via an N-methyl-D-aspartate receptor-dependent mechanism. J Neurosci. 1994; 14:7680–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Di Paolo T. Modulation of brain dopamine transmission by sex steroids. Rev Neurosci. 1994; 5:27–41. 10.1515/revneuro.1994.5.1.27 [DOI] [PubMed] [Google Scholar]
- 39.Karakaya S, Kipp M, Beyer C. Oestrogen regulates the expression and function of dopamine transporters in astrocytes of the nigrostriatal system. J Neuroendocrinol. 2007; 19:682–90. 10.1111/j.1365-2826.2007.01575.x [DOI] [PubMed] [Google Scholar]
- 40.Castle DJ, Abel K, Takei N, Murray RM. Gender differences in schizophrenia: hormonal effect or subtypes? Schizophr Bull. 1995; 21:1–12. 10.1093/schbul/21.1.1 [DOI] [PubMed] [Google Scholar]
- 41.Kulkarni J. Women and schizophrenia: a review. Aust N Z J Psychiatry. 1997; 31:46–56. 10.3109/00048679709073798 [DOI] [PubMed] [Google Scholar]
- 42.Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987; 44:660–69. 10.1001/archpsyc.1987.01800190080012 [DOI] [PubMed] [Google Scholar]
- 43.Häfner H, Maurer K, Löffler W, Riecher-Rössler A. The influence of age and sex on the onset and early course of schizophrenia. Br J Psychiatry. 1993; 162:80–86. 10.1192/bjp.162.1.80 [DOI] [PubMed] [Google Scholar]
- 44.Hiraoka M, Kagawa Y. Genetic polymorphisms and folate status. Congenit Anom (Kyoto). 2017; 57:142–49. 10.1111/cga.12232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mandaviya PR, Stolk L, Heil SG. Homocysteine and DNA methylation: a review of animal and human literature. Mol Genet Metab. 2014; 113:243–52. 10.1016/j.ymgme.2014.10.006 [DOI] [PubMed] [Google Scholar]
- 46.Trześniowska-Drukała B, Kalinowska S, Safranow K, Kłoda K, Misiak B, Samochowiec J. Evaluation of hyperhomocysteinemia prevalence and its influence on the selected cognitive functions in patients with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2019; 95:109679. 10.1016/j.pnpbp.2019.109679 [DOI] [PubMed] [Google Scholar]
- 47.Moustafa AA, Hewedi DH, Eissa AM, Frydecka D, Misiak B. Homocysteine levels in schizophrenia and affective disorders-focus on cognition. Front Behav Neurosci. 2014; 8:343. 10.3389/fnbeh.2014.00343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dhonukshe-Rutten RA, de Vries JH, de Bree A, van der Put N, van Staveren WA, de Groot LC. Dietary intake and status of folate and vitamin B12 and their association with homocysteine and cardiovascular disease in european populations. Eur J Clin Nutr. 2009; 63:18–30. 10.1038/sj.ejcn.1602897 [DOI] [PubMed] [Google Scholar]
- 49.Waśkiewicz A, Piotrowski W, Broda G, Sobczyk-Kopcioł A, Płoski R. Impact of MTHFR C677T gene polymorphism and vitamins intake on homocysteine concentration in the polish adult population. Kardiol Pol. 2011; 69:1259–64. [PubMed] [Google Scholar]
- 50.Zhang DM, Ye JX, Mu JS, Cui XP. Efficacy of vitamin B supplementation on cognition in elderly patients with cognitive-related diseases. J Geriatr Psychiatry Neurol. 2017; 30:50–59. 10.1177/0891988716673466 [DOI] [PubMed] [Google Scholar]
- 51.Jurinke C, Oeth P, van den Boom D. MALDI-TOF mass spectrometry: a versatile tool for high-performance DNA analysis. Mol Biotechnol. 2004; 26:147–64. 10.1385/MB:26:2:147 [DOI] [PubMed] [Google Scholar]