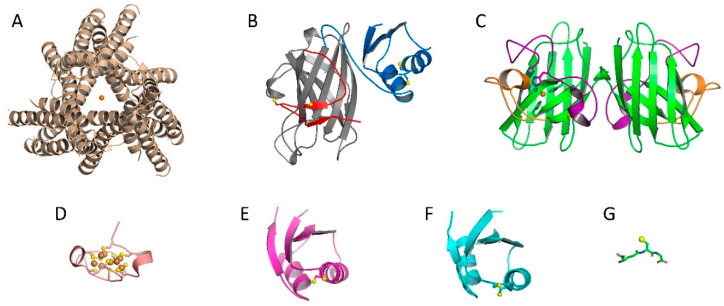
Figure 1.
A collection of copper-binding molecules relevant to copper acquisition, regulation, and distribution to Sod1. (A) The copper importer Ctr1 with copper (orange sphere) bound in the channel (PDB: 6M98). (B) The structure of yeast Ccs, complete with D1 (blue), D2 (gray), and D3 (red). Copper binding cysteines are shown as yellow spheres (PDB: 5U9M). (C) Mature Sod1 dimer with the β-barrel shown in green and critical loop elements in purple (zinc loop) and orange (electrostatic loop). Active site bound copper is displayed as an orange sphere and the adjacent zinc shown in grey (PDB: 1PU0). (D) Copper bound MT3 domain with the coppers as orange spheres and the coordinating cysteines as yellow spheres (PDB: 1RJU). (E) The copper chaperone Atox1 (monomer) with the MTCxxC cysteines shown as yellow spheres (PDB: 5F0W). (F) A copper-binding domain (repeat 2) of the transport protein ATP7B, again with the conserved MTCxxC cysteines shown as yellow spheres (PDB: 2LQB). Notice the structural similarities between Ccs D1, Atox1, and ATP7B. (G) The structure of the glutathione tri-peptide, with the cysteine shown as a yellow sphere (PDB: 1AQW).