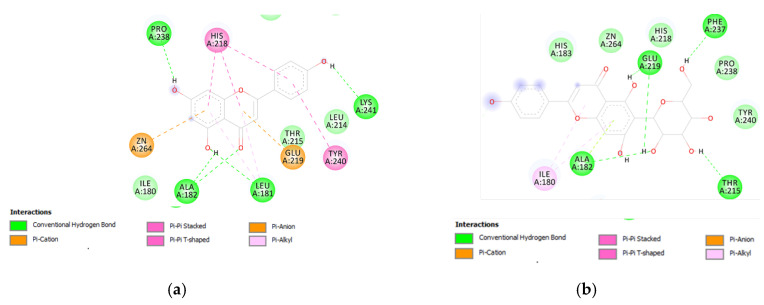
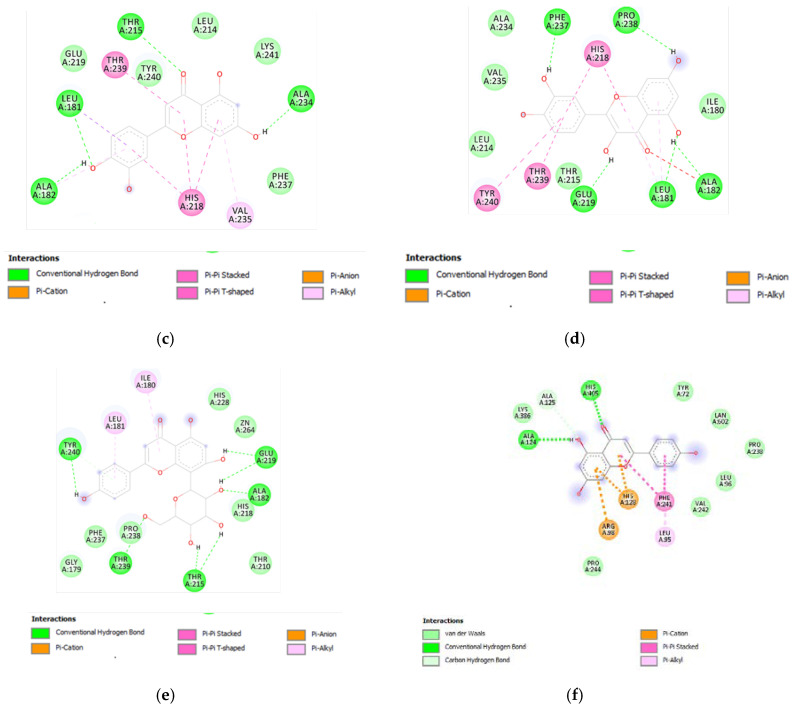
Figure 8.
(a) Putative interactions between apigenin and MMP-12 (PDB: 3F17). Free energy of binding (ΔG) and affinity (Ki) are −8.3 kcal/mol and 0.8 µM, respectively. (b) Putative interactions between isovitexin and MMP-12 (PDB: 3F17). Free energy of binding (ΔG) and affinity (Ki) are −8.8 kcal/mol and 0.4 µM, respectively. (c) Putative interactions between luteolin and MMP-12 (PDB: 3F17). Free energy of binding (ΔG) and affinity (Ki) are −8.7 kcal/mol and 0.4 µM, respectively. (d) Putative interactions between quercetin and MMP-12 (PDB: 3F17). Free energy of binding (ΔG) and affinity (Ki) are −8.7 kcal/mol and 0.4 µM, respectively. (e) Putative interactions between vitexin and MMP-12 (PDB: 3F17). Free energy of binding (ΔG) and affinity (Ki) are −8.8 kcal/mol and 0.3 µM, respectively. (f) Putative interactions between apigenin and lanosterol-14-alpha demethylase (PDB: 4LXJ). Free energy of binding (ΔG) and affinity (Ki) are −8.8 kcal/mol and 0.4 µM, respectively.