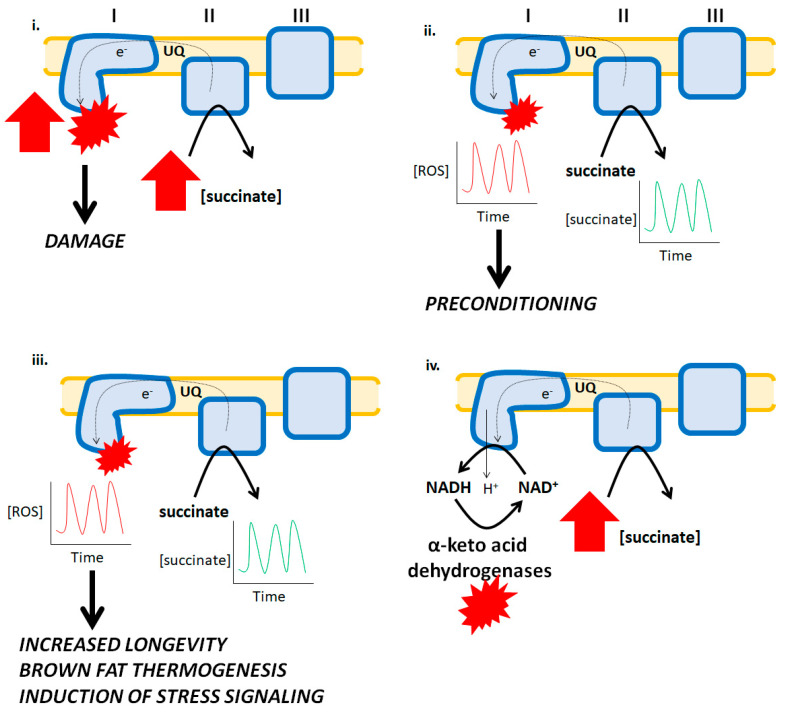
Figure 2.
Reverse electron transfer (RET) and its pathological effects and physiological benefits. i. The accumulation of succinate during ischemia results in a high burst in ROS production by complex I. Succinate is oxidized by complex II and due to the hyperpolarization of the mitochondrial inner membrane and over-reduction of UQ pools, electrons flow backwards to complex I forming high amounts of ROS culminating with myocardial tissue damage and the development of heart disease. ii. Ischemic preconditioning is associated with temporary bursts in ROS production by mitochondria which activates cellular programs involved in adaptative signaling resulting in a bolstering of antioxidant systems that protect cardiomyocytes from oxidative stress. In this hypothetical scenario, the short bursts in ROS production are caused by spatio-temporal increases and decreases in succinate, which results in short and temporary bursts in ROS production by RET. iii. Increases and decreases in succinate availability are integral for driving RET-mediated ROS production in brown fat and other tissues, contributing to vital physiological functions such as thermogenesis, stress signaling, and longevity. iv. A hypothetical mechanism for how RET from succinate to complex I drives ROS production by α-keto acid dehydrogenases. Reverse electron flow drives proton return to the matrix by complex I resulting in NADH formation and its subsequent oxidation by the dehydrogenases. This leads to ROS production by these enzymes.