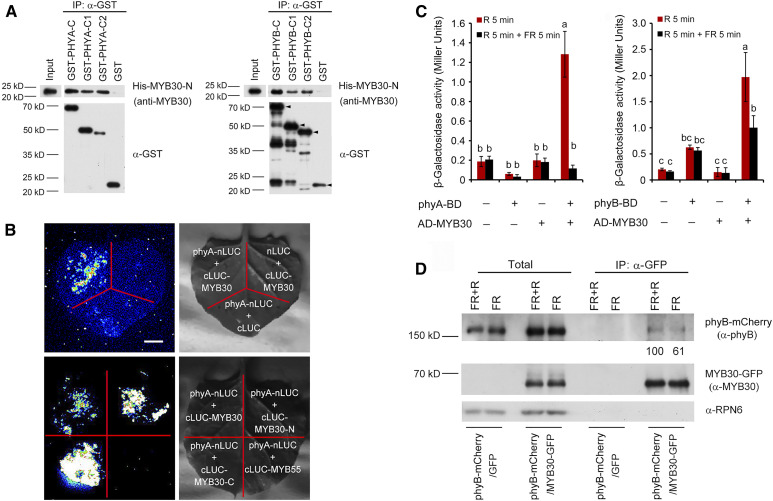
Figure 1.
MYB30 Directly Interacts with phyA and phyB.
(A) In vitro pull-down assay to test for phyA and phyB interaction with MYB30. Assays used PHYA-C, PHYA-C1, and PHYA-C2 (left) or PHYB-C, PHYB-C1, and PHYB-C2 (right) with the R2R3-MYB domain of MYB30 (MYB30-N). His-tagged MYB30-N proteins pulled down with GST-PHYA/B-C, GST-PHYA/B-C1, GST-PHYA/B-C2, or GST were detected using anti-MYB30 antibodies. The arrowheads in the left three lanes of the PHYB panel indicate the positions of full-length GST-tagged proteins. Input, 6% of the purified His-tagged target proteins used in pull-down assays.
(B) LCI assays using phyA-nLuc and cLuc-MYB30 fusions in N. benthamiana leaf cells. An interaction was seen between phyA-nLUC and cLUC-MYB30, but not with the negative controls lacking phyA or MYB30 (top panels). Specifically, phyA interacted with both N- and C-terminal domains of MYB30, but not with MYB55 (bottom panels). Bar = 1 cm.
(C) GAL4 yeast two-hybrid assays showing that MYB30 preferentially interacted with the Pfr forms of both phyA and phyB. Yeast cells transformed with the indicated plasmids were used for ONPG assays. The yeast cultures were irradiated either with 5 min of R light (60 µmol m−2 s−1) alone or with 5 min of R light immediately followed by 5 min of FR light (40 µmol m−2 s−1), and cultures were then incubated for 2 h. The yeast cultures were exposed to the same R or R + FR light treatments again and incubated for another 2 h. The β-galactosidase activities were then measured by liquid culture assays using ONPG as the substrate. Error bars represent sd of three independent yeast cultures. Different letters represent statistical significances determined by ANOVA with Tukey’s post hoc test (P < 0.05; Supplemental Data Set 3).
(D) Co-IP assays showing that MYB30 preferentially interacted with the Pfr form of phyB in vivo. phyB-mCherry and MYB30-GFP fusion proteins were expressed in Arabidopsis protoplasts. After extraction, proteins were exposed to 5 min of FR light (40 µmol m−2 s−1) or 5 min of FR light immediately followed by 5 min of R light (60 µmol m−2 s−1) and then incubated with GFP-trap agarose beads. Total (left side) and precipitated (right side) proteins were analyzed by immunoblotting using antibodies against phyB (top), MYB30 (middle), and RPN6 (bottom).