Abstract
Plant ATP-binding cassette (ABC) I subfamily (ABCIs) consists of heterogeneous members of proteins which have any of the domains found in other ABC proteins. Some ABCIs have critical functions in basic metabolism and stress responses. However, many ABCI proteins remain functionally uncharacterized. ABCI19, ABCI20, and ABCI21 of Arabidopsis thaliana (ABCI19/20/21) cluster together in phylogenetic tree, and were suggested as targets of ELONGATED HYPOCOTYL 5 (HY5). In this study, we reveal that the three ABCIs are involved in modulation of cytokinin response during early seedling development. ABCI19, ABCI20 and ABCI21 promoters harbor HY5 binding motifs, and the expression of ABCI20 and ABCI21 was induced by light in a HY5-dependent manner. The triple abci19 abci20 abci21 and the double abci20 abci21 knockouts exhibited hypersensitive seedling growth retardation in the presence of exogenous cytokinin, but exhibited no phenotypic difference from the wild type either in control medium or in auxin-, ABA-, GA-, ACC- or BL-containing media. ABCI19, ABCI20, and ABCI21 were expressed in young seedlings and the proteins interacted with each other, forming a large protein complex at ER membrane. These results suggest that ABCI19, ABCI20, and ABCI21 fine-tune cytokinin response at ER under the control of HY5 at young seedling stage.
Keywords: ABC transporter, cytokinin, HY5, seedling growth, endoplasmic reticulum
Introduction
Plant ATP-binding cassette transporters (ABC transporters) transport numerous substrates necessary for biotic or abiotic stress resistance, growth, and development (Kang et al. 2011; Do et al. 2018). Most ABC transporters have both nucleotide-binding domain (NBD) and transmembrane domain (TMD) for their transport functions. However, there are non-intrinsic ABC (ABCI) proteins, which have only NBD, TMD or accessary components of prokaryotic multi-subunit ABC transporters (Verrier et al. 2008). Arabidopsis encodes 21 ABCI proteins, and some of them play critical functions in basic metabolism and stress responses. For example, ABCI1 and ABCI2 constitute Cytochrome c maturation (CCM) complex, which attaches the heme prosthetic group to apocytochrome c at the mitochondrial inner membrane (Rayapuram et al. 2007). ABCI6, ABCI7, and ABCI8 are components of Fe-S cluster biogenesis (ISB) complex located at plastid, and thus are important for the biogenesis of cytochrome b6/f complex, ferredoxin and photosystem I (Xu et al. 2004). ABCI13 (TGD3), ABCI14 (TGD1) and ABCI15 (TGD2) form Trigalactosyldiacylglycerol (TGD) complex (Roston et al. 2012; Wang et al. 2013; Fan et al. 2015) which transports phosphatidic acid (PA) from ER to chloroplast. ABCI16 (ALS3, TMD) and ABCI17 (STAR1, NBD) play a role in tolerance mechanism to Al stress (Larsen et al. 2005; Huang et al. 2010). Other ABCI proteins might also play critical functions in plants, but remain unknown.
Light is one of the most important signals for plant development, and light signal transduction pathway consists of thousands of genes regulated by numerous transcription factors. HY5 is one of the best-known transcription factors that are induced by light. It is a basic-leucine zipper (bZIP) transcription factor, and regulates photomorphogenesis by inducing expression of light-responsive genes (Gangappa et al. 2016; Chang et al. 2008; Ang et al. 1998; Chattopadhyay et al. 1998; Koornneef et al. 1980) and via crosstalk with various phytohormones including cytokinin (Chen et al. 2008; Rasmussen et al. 2012; Xu et al. 2014). A previous study attempted to identify the direct targets of HY5, using DNA chip hybridization combined with a high-density 60-nucleotide oligomer microarray (Lee et al. 2007). The study revealed more than 3,000 chromosomal sites as putative HY5 binding targets, and the list of the sites included three ABCI sub-family members of ABC proteins.
Here, we focused our research on ABCI19, ABCI20, and ABCI21, the three ABCI subfamily proteins that have been predicted to be regulated by HY5, but had hitherto not been studied in detail. We found that abci19 abci20 abci21 triple knockout and abci20 abci21 double knockout seedlings exhibited an increased sensitivity to exogenous cytokinin (trans-zeatin), but not to other major plant hormones. We also found that ABCI20 and ABCI21 expression was induced by light in a manner dependent on HY5. Together, our data suggest that the three ABCI proteins fine tune the cytokinin response during photomorphogenesis at seedling stage of Arabidopsis.
Materials and methods
Plant growth conditions
A. thaliana ecotype Col-0 and transgenic plants were grown on half-strength Murashige and Skoog (1/2 MS) agar media with 1% sucrose in the absence or presence of the indicated phytohormones. The seedlings were grown in a growth chamber under a 16-hr light/8-hr dark cycle at 22 °C.
Generation of phylogenetic tree of ABCI subfamily
The protein sequence of NBD-type ABCI proteins was obtained from TAIR (https://www.arabidopsis.org/) and used to generate a phylogenetic tree using CLUSTALW program (https://www.genome.jp/tools-bin/clustalw). The midpoint rooted tree was generated using the protein sequence of ABCI proteins. The possible domains of ABCI proteins were analyzed using public database (InterPro; https://www.ebi.ac.uk/interpro/; Mitchell et al. 2019), and we used Arabidopsis ABCI protein nomenclature used previously (Verrier et al. 2008).
Generation and isolation of abci19, abci20, and abci21 mutants
T-DNA insertion mutant of ABCI21 (AT5G44110; abci21, SALK_064144) was obtained from the Arabidopsis Biological Resource Center (http://abrc.osu.edu/). T-DNA border-specific primers were used to confirm T-DNA insertion, and RT-PCR using gene-specific primers verified that abci21 was a null mutant. To knockout ABCI20 (AT5G02270), CRISPR/Cas9-mediated genome editing method was used (Gao et al. 2014). Genome editing in abci20 mutants was confirmed by PCR with ABCI20-GT2 and ABCI20-GT3 primers and sequential restriction enzyme digestion with Bsl I for abci20–1 mutation. abci20–2 mutation was confirmed using PCR with ABCI20-GT1 and ABCI20-GT2 and Xmn I restriction enzyme digestion. abci21 was crossed with abci20–1 and abci20–2 to generate abci20–1 abci21 (abci20–1/21) and abci20–2 abci21 (abci20–2/21) double knockout mutants, respectively. To generate abci19 abci20 abci21 triple knockout mutants, a gene fragment in ABCI19 (AT1G03905) was deleted from abci20–1 abci21 double knockout mutant, following the method described in Gao et al. (2016). ABCI19-GT1 and ABCI19-GT2 primers were used to check the gene fragment deletion using PCR. The primer sequence information is available in Supplemental Table 1.
ABCI20 promoter:GUS Expression Assay
A region 2-kb upstream of the AtABCI20 start codon was PCR-amplified from genomic DNA and cloned into pMDC163 (Curtis et al. 2003). GUS analysis was performed with T3 plants at different stages of development as described in Kim et al. (2009). One day-after-sowing (DAS) the seeds, 3, 7, or 14 DAS seedlings, and flowers were incubated for 4 hours in GUS solution containing X-Gluc (5-bromo-4-chloro-3-indolyl-β-O-glucopyranoside) at 37 °C for GUS staining observation.
Analysis of Transcript Levels
Total RNA was isolated from Arabidopsis seedlings using Takara RNAiso Plus reagent (TAKARA, http://www.takara-bio.com/). Quantitative real-time PCR was performed using the TB Green® Premix Ex Taq™ Tli RNase H Plus (TAKARA, http://www.takara-bio.com/), following the manufacturer’s instructions. The relative abundance of each cDNA was normalized against TUB8 (AT5G23860) or ACT1 (AT2G37620). The sequences of gene-specific primers are provided in Supplemental Table 1. Primers used to detect HY5 (AT5G11260) transcripts were the same as reported previously (Favory et al. 2009).
Measurement of root apical meristem size
Arabidopsis seedlings were grown on ½ MS-agar media supplemented with trans-zeatin for 8 days, stained with propidium iodide (10 mg/ml), and imaged with confocal laser scanning microscopy (CLSM) (excitation at 564 nm and emission at > 570 nm). Cortical cells that do not exhibit obvious cell elongation, with the cell length is shorter than 20 μm, were counted (Hwang et al. 2018).
TCS:GFP observation
Fluorescence of TCS:GFP was observed in 8-day-old seedlings (T2) of wild types or abci20–1/21 expressing TCS:GFP using Olympus FLUOVIEW FV1000 confocal laser scanning microscope system. Excitation and emission were at 488 nm, and 520 nm, respectively. Fluorescent intensity was measured in the root tip and calculated using Image J (https://imagej.nih.gov/ij/index.html; Schneider et al. 2012). The background fluorescence intensity was subtracted from the total intensity, following the method described previously (Ali et al. 2019).
Subcellular Localization of ABCI20 and ABCI21
To observe ABCI20:GFP localization in A. thaliana roots, A 3.7-kb fragment of genomic DNA containing the ABCI20 promoter region and the ABCI20 open reading frame was cloned into pMDC107 (proABCI20:ABCI20 genomic DNA fragment:(Ala)7:GFP), and stably introduced to A. thaliana plants. The coding sequence (CDS) of ABCI20 in fusion to the YFP CDS and BiP:RFP were introduced to Arabidopsis protoplasts using PEG transformation method (Lee et al. 2005). Fluorescence of YFP:ABCI20 and BiP:RFP was observed using Olympus FLUOVIEW FV1000 confocal laser scanning microscope, 32 hours after the transformation. For the transient expression in tobacco leaves, CDS of ABCI21 in fusion to RFP was cloned into pMDC32 plasmid (Curtis et al. 2003) and introduced to tobacco leaves using Agrobacterium infiltration method (Sheikholeslam et al. 1987). Fluorescence of YFP was observed 48 hours after Agrobacterium infiltration. To test co-localization of ABCI20 and ABCI21, pMDC107:proABCI20:ABCI20 genomic DNA fragment:(Ala)7:GFP and pMDC32:pro35S:RFP:ABCI21 were co-infiltrated into tobacco leaves. Fluorescence of ABCI20:GFP and RFP:ABCI21 was observed 72 hours after co-infiltration using Olympus FLUOVIEW FV1000 confocal laser scanning microscope.
Subcellular Membrane Fractionation
For subcellular fractionation, 2-week-old plants were homogenized. Centrifugation, fractionation, sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and immunoblotting were performed as described previously (Kobae et al. 2006).
Anti-ABCI20 serum generation and purification of anti-ABCI20 antibody
Peptide antigen of ABCI20, RTEESRVTGDPARMLN (from 311–326 aa of ABCI20), was synthesized (YOUNG IN FRONTIER, https://www.younginfrontier.com/laboratory/index.php). We boost rabbits for 4 times by injecting the peptide antigen. Anti-ABCI20 antibody was purified from the immunized rabbit serum with Affi-Gel 15 resin following manufacturer’s instructions (BIO-RAD). Purified anti-ABCI20 antibody was suspended in PBS buffer for further uses.
Analysis of ABCI20 interacting partners
Total proteins were extracted from 8-day-old wild type or abci20–1/21 seedlings using the protein extraction buffer (50 mM Tris-HCl pH 8, 10 mM KCl, 10 mM MgCl2, 1 mM EDTA, 20% Glycerol (v/v), 0.25% Triton X-100 (v/v), Pierce™ protease inhibitor tablet EDTA-free). The total protein extracts were incubated with anti-ABCI20 antibody for overnight at 4°C and with Affi-Gel 15 (BIO-RAD) for overnight at 4°C. The mixture of total proteins, anti-ABCI20, and resin was placed to the Poly-Prep® Chromatography Columns (BIO-RAD) and sequentially rinsed with 100 mM, 200 mM, 500 mM, and 1 M NaCl/Tris-HCl buffers (twice per each). ABCI20 and associated proteins were finally eluted using the elution buffer (8 M Urea, 0.1 M NaH2PO4 pH 8, 50 mM Tris-HCl pH 8). The eluted proteins were separated by SDS-PAGE and further analyzed by liquid chromatography hybrid-FT orbitrap mass spectrometry (Korea basic science institute, Ochang, Korea). The results of LTQ-MS are listed in Supplemental Table 2.
Native PAGE
Total proteins from the 7-day-old wild types and abci20–1/21 seedlings were extracted and separated using blue native PAGE as previously described (Yoo et al. 2016).
Results
ABCI19, ABCI20, and ABCI21 are likely to be functionally related
To understand how the three ABCI proteins are related, we first constructed a phylogenetic tree of Arabidopsis thaliana ABCI proteins based on their amino acid sequences. Since ABCI19, ABCI20, and ABCI21 contain only NBD but no TMD, we included in the tree only the NBD-containing ABCI members. The three ABCIs formed a cluster distinct from other NBD-type ABCI proteins, which suggested that they are closely related (Fig. 1a). Indeed, ABCI19 and ABCI21 have high similarity to ABCI20 in amino acid sequence; ABCI19 has 57% identity and 72% similarity; ABCI21 has 52% identity and 64% similarity. There was no distinct domain of proteins identifiable from the three ABCI proteins, except for the NBDs, which, in ABC proteins, most often bind ATP and function as ATPases.
Fig. 1.
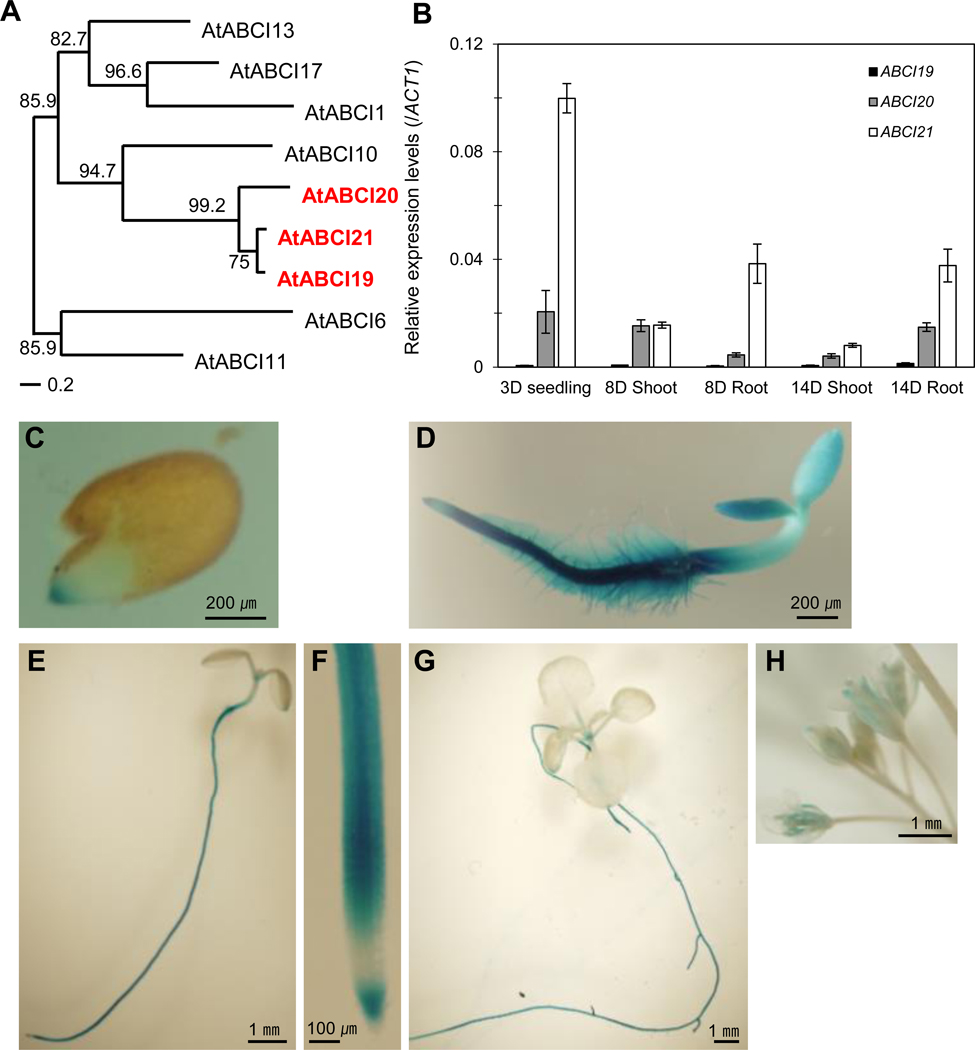
ABCI19, ABCI20, and ABCI21 are closely related NBD-type ABC proteins expressed at young seedling stages.
(a) Phylogenetic tree of 9 NBD-type ABCI proteins in A. thaliana. Protein sequences were aligned using ClustalW program (http://www.genome.jp/tools/clustalw/), and then phylogenetic analysis was undertaken to produce a midpoint rooted tree. ABCI19, ABCI20 and ABCI21 are marked in red.
(b) Relative expression levels of ABCI19, ABCI20 and ABCI21 measured by qRT-PCR at seedling stage after 3–14 days of seed sowing. Data were normalized using the expression level of ACTIN1. Mean values (±SD) of two independent experiments (n=3) are given.
(c-h) The GUS signals of proABCI20:GUS transgenic plants observed in 1-day-old (c), 3-day-old (d), 7-day-old (e and f), and 2-week-old (g) seedlings and in flowers (h).
To get hints on functional roles of ABCI19, ABCI20, ABCI21, we examined their tissue-specific expression patterns. We first quantified the transcript levels of ABCI19, ABCI20 and ABCI21 using quantitative real-time PCR (qRT-PCR). In early seedling stages of 3–8 days after seed sowing, ABCI19, ABCI20, and ABCI21 were expressed in both shoot and root, and ABCI19 transcript level was much lower than those of ABCI20 and ABCI21 (Fig. 1b). The expression of ABCI19, ABCI20 and ABCI21 became mostly root-selective at 14 DAS (Fig. 1b). To observe the tissue-specific expression pattern of ABCI20 in more detail, we generated Arabidopsis plants expressing a GUS reporter transcriptionally fused to ABCI20 promoter (proABCI20:GUS). Consistent with qRT-PCR results (Fig. 1b), proABCI20:GUS activity was strong in the root, especially at the tip during germination and throughout the seedling stage (Fig. 1c–g). The shoot parts, including anthers in flower, expressed this gene, but not as strongly as the root (Fig. 1e–h). ABCI21 was shown to be specifically expressed in root of light-grown seedlings in a study using ABCI21 promoter-GUS reporter lines (Marin et al. 2006). In addition, ABCI21 is ranked high in the co-expression gene list of ABCI20 (Supplemental Table 2). Taken together, these results suggest that ABCI20 and ABCI21, and possibly ABCI19 too, might have overlapping functions at the seedling stage of development.
abci19 abci20 abci21 triple and abci20 abci21 double knockout mutants are hypersensitive to cytokinin
Since we suspected that the three ABCI proteins have overlapping functions, we reasoned that any single gene knockout of them may not produce detectable phenotypic changes. Therefore, we generated double and triple knockout mutations of ABCI19, ABCI20, and ABCI21. First, we generated abci20 mutations using the CRISPR/Cas9-mediated genome editing technology (Gao et al. 2014). Nucleotide substitutions in ABCI20 exons resulted in premature stop codons and knocked out ABCI20 (Fig. 2a). Next, we obtained abci21 mutant (SALK_064144) from ABRC (Arabidopsis Biological Resource Center https://abrc.osu.edu/). In the abci21 mutant, T-DNA was inserted in the last exon of ABCI21, knocking out ABCI21 expression (Fig. 2b). Third, we generated abci20 abci21 double mutants by crossing abci20–1 and abci20–2 with abci21 mutant. Finally, we introduced genome deletions of ABCI19 into abci20–1 abci21 double mutant, following the method described in Gao et al. (2016). Normal size (2,629 bp) of amplicons of ABCI19 was not detected in abci19 abci20 abci21 mutants (Fig. 2c).
Fig. 2.
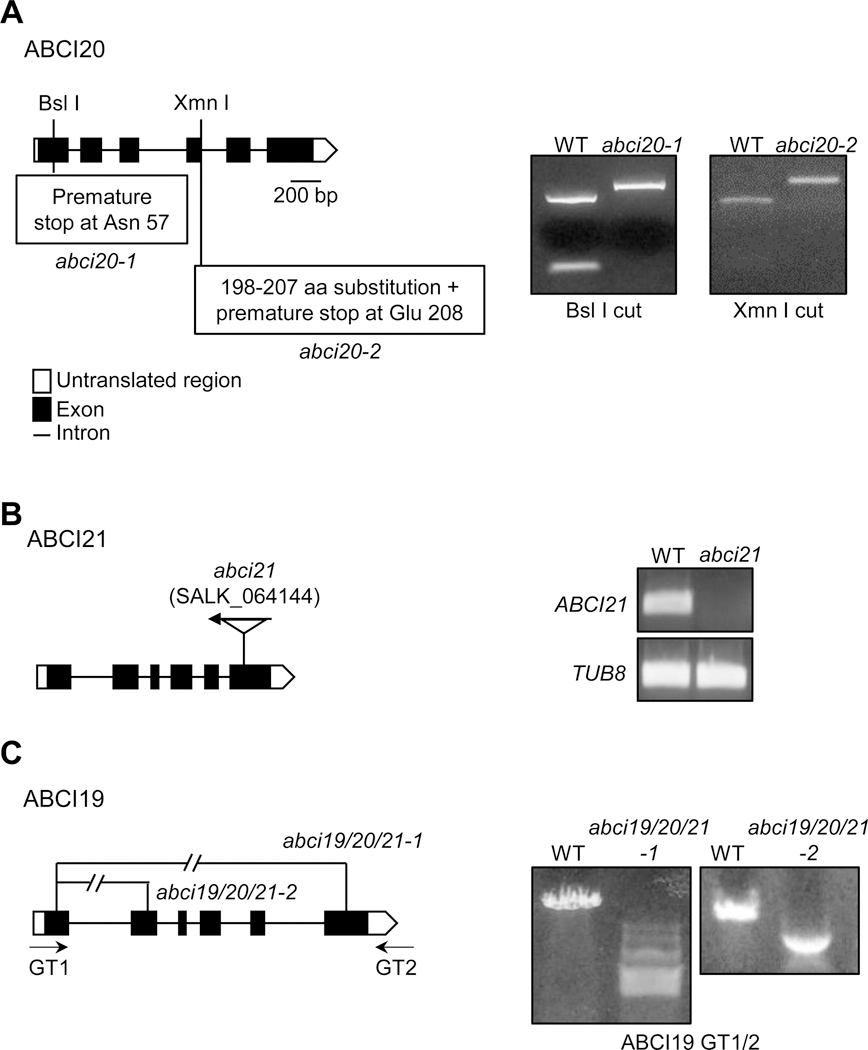
Generation of abci19 abci20 abci21 knockout mutants.
The locations of gene fragment deletions, point mutation, or T-DNA insertion in the abci19 abci20 abci21 mutants are presented.
(a) The premature stop codons and amino acid substitution in abci20–1 and abci20-2 produced by CRISPR/Cas9. PCR-amplified genomic DNA of the mutants were resistant, whereas the wild type PCR product was digested successfully by the restriction enzymes Bsl I and Xmn I.
(b) T-DNA insertional position in abci21 (SALK_064144). RT-PCR analysis confirmed the absence of ABCI21 transcript in abci21 mutant.
(c) Two sets of guide RNA pairs targeting ABCI19 gene produced two different fragment deletions in ABCI19 in the background of abci20–1 abci21. PCR using GT1/2 primers confirmed ABCI19 gene deletion.
The seedling growth of abci19 abci20 abci21 triple mutants was not significantly different from that of the wild type in control growth condition (Fig. 3a, b); the biomass of shoot and root was only slightly reduced (Fig. 3b, c and Figs. S1 and S2). We next examined the possibility that ABCI19, ABCI20, and ABCI21 are involved in regulation of phytohormone responses during seedling growth on 1/2 MS-agar media containing various phytohormones. Tested hormones included cytokinin (trans-zeatin), gibberellic acid, IAA, abscisic acid, ACC (ethylene precursor) and brassinosteroid, and they were used at concentrations reported to induce obvious growth changes (Kudo et al. 2012; Zhong et al. 2015; Zhong et al. 2016; Okumura et al. 2013; Chen et al. 2008; Sun et al. 2015; Tanaka et al. 2003). abci19 abci20 abci21 triple knockout seedlings exhibited an enhanced sensitivity to trans-zeatin (tZ) compared to the wild type (Fig. 3); on 10~50 nM tZ-containing media, the abci19 abci20 abci21 triple mutants were more inhibited in growth of shoot and primary roots than the wild type. In contrast, in the presence of 0.2 μM IAA, 1 μM ABA, 1 μM ACC, or 1 nM BL, the growth of the triple mutants was not significantly different from that of the wild type (Fig. S1). Neither was there any obvious difference between abci19 abci20 abci21 and the wild type in seed germination in the presence of 1 μM GA (Fig. S1d). To understand the basis of the shorter primary root in the presence of tZ (Fig. 3a, c), we counted the number of cortical cells in root apical meristem (RAM) from the images of longitudinal median sections of root tips stained with propidium iodide. The number of the cortical cells of RAM was significantly less in abci19 abci20 abci21 than in the wild type in the presence of trans-Zeatin (Fig. 3d).
Fig. 3.
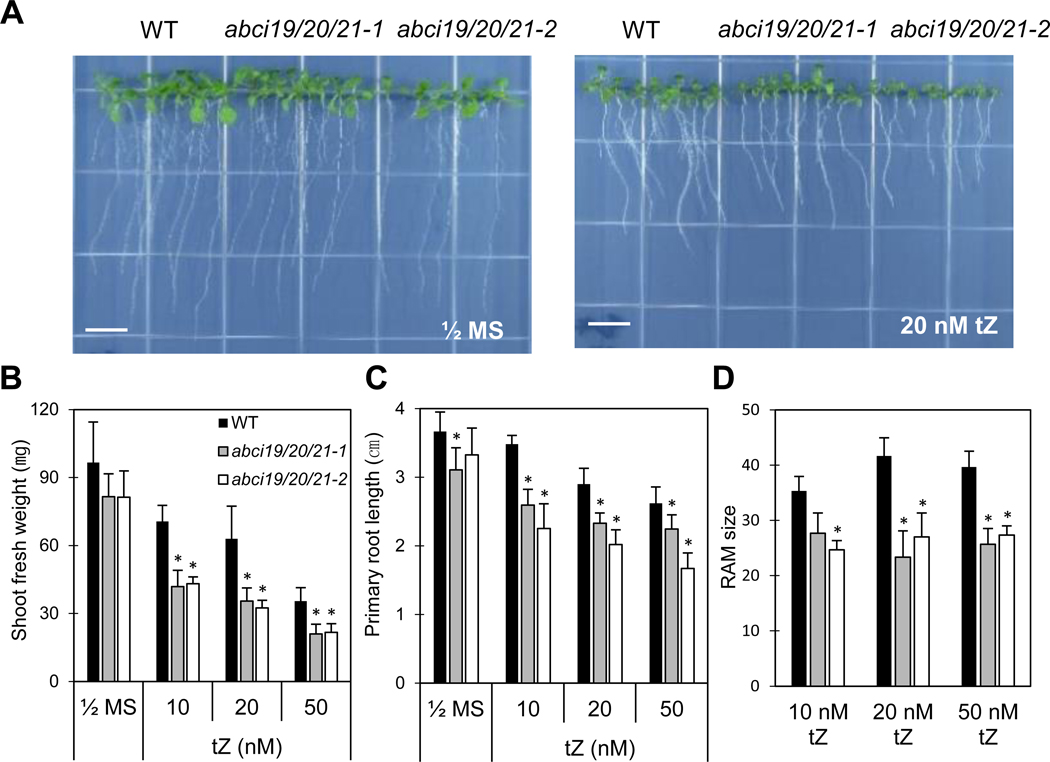
Cytokinin-hypersensitive seedling growth of abci19 abci20 abci21 mutants.
(a) Plants were vertically grown on ½ MS-agar plates in the absence or presence of trans-zeatin. Pictures were taken at 12 days after seed sowing. Scale bar = 1 cm.
(b, c) Plant growth under conditions given in (a) quantified by measuring the total shoot fresh weight per plate and the primary root length. Mean values (±SD) of two independent experiments (3 biological replicates each) are presented (*; p < 0.03, Student’s t test).
(d) The numbers of cortical cells in RAM under trans-zeatin treatment condition. The wild type and mutants were grown on 1/2 MS-agar media containing 10, 20, or 50 nM trans-zeatin for 8 days. Mean values (±SD) are presented (*; p < 0.03, Student’s t test, N=2, n=6).
We also examined abci20 single knockout and abci20 abci21 double mutants whether they exhibited similar cytokinin-sensitive phenotypes as abci19 abci20 abci21 did (Fig. 3a). A single knockout of ABCI20, abci20–1, did not exhibit any phenotype under control or cytokinin-treated conditions (Fig. S2). In contrast, abci20 abci21 double mutants exhibited shorter roots, smaller shoots, and more reduced RAM than the wild type in the presence of tZ (Fig. S3). However, the extent of their phenotypes was slightly less than those of the triple phenotypes (Fig. S3e). The small effect of additional knockout of ABCI19 in the background of abci20 abci21 might be because ABCI19 expression level was much lower than ABCI20 or ABCI21 (Fig. 1b). Together, these results suggest that ABCI19, ABCI20 and ABCI21 play overlapping functions in cytokinin response at seedling stage.
Cytokinin signaling is enhanced in abci20 abci21
We next examined whether cytokinin signaling was enhanced in plants without expression of ABCI20 and ABCI21. For this purpose, we used a synthetic reporter of the two component signaling sensor (TCS) of cytokinin: green fluorescent protein (GFP), which reflects the transcriptional activity of type-B Arabidopsis response regulators (Sakai et al. 2000; Hosoda et al. 2002; Imamura et al. 2003). This reporter enabled visualization of cytokinin signaling in vivo (Muller et al. 2008). We introduced the TCS:GFP-reporter to the wild-type and abci20–1 abci21 plants and assessed the intensity levels of GFP fluorescence in root tips of multiple independent transgenic plants (Fig. 4). Because TCS:GFP basal expression level was different between the lines, we measured the total TCS:GFP intensity values from median sectioned CLSM images of root tips, and calculated fold change of GFP intensity values in tZ-treated seedlings (+ tZ) over those in non-treated control seedlings (− tZ). The fold increases in TCS:GFP fluorescence levels in 20 nM tZ-containing medium over in control medium were higher in abci20–1 abci21 double mutant background lines than in the wild type background lines (Fig. 4b). This result indicated that cytokinin response was indeed higher in abci20–1 abci21 than in the wild type, at least in the roots, and suggested that ABCI20 and ABCI21 suppress cytokinin response.
Fig. 4.
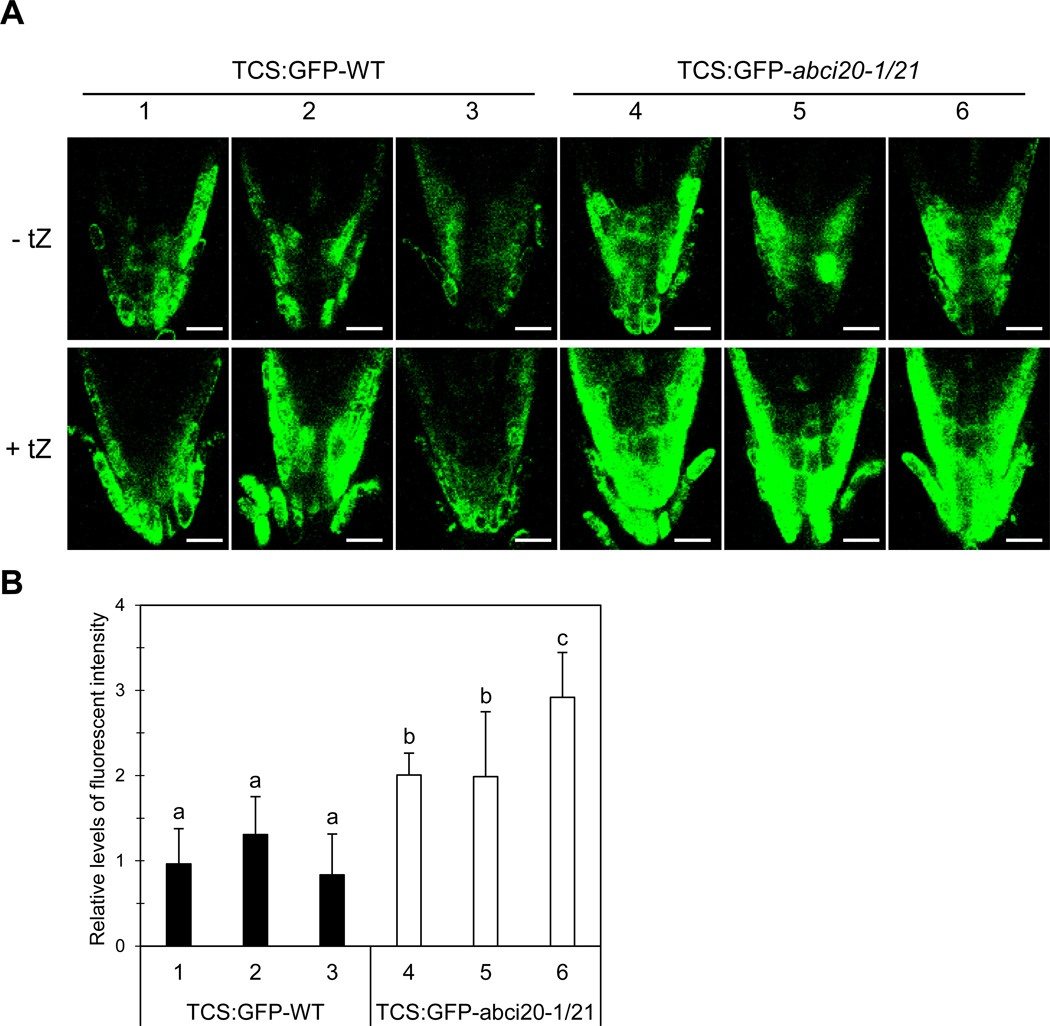
Enhanced cytokinin signaling in abci20 abci21.
(a) Representative root tip images of multiple TCS:GFP-expressing wild type and abci20–1/21 lines grown in the absence or presence of 20 nM tZ. Scale bar = 20 μm.
(b) TCS:GFP fluorescence intensity measured in the root tips of the wild type or abci20–1/21 transgenic lines expressing TCS:GFP. Seeds were germinated and grown on ½ MS-agar media supplemented with or without 20 nM trans-zeatin. Images were taken at 8 days after seed sowing. The fold increase of TCS:GFP level in tZ-treated root tips over in non-treated control root tips was calculated. The combined results (mean ± SD) of two independent experiments (total 7 biological replicates) are given. Different alphabets indicate that the means are significantly different between genotypes by Tukey’s Honest Significant Difference test at p ≤ 0.01.
ABCI20 and ABCI21 are localized at the endoplasmic reticulum
To get hints on the cellular functions of ABCI20 and ABCI21, we investigated the intracellular localization of the two proteins by generating plants expressing the proteins fused with fluorescent proteins. First, we stably expressed GFP-fused ABCI20 in A. thaliana seedlings, and observed where ABCI20-GFP was localized in root tip cells (Fig. 5a). ABCI20-GFP signal was distributed in endomembrane-like structures (zoomed images in Fig. 5a). We next transiently co-expressed ABCI20-YFP and Bip-RFP, a marker of the ER membrane, in Arabidopsis leaf protoplasts. ABCI20-YFP signal was co-localized with the ER marker (Fig. S4). To further investigate the exact subcellular localization of ABCI20, we extracted the total proteins from ABCI20-GFP expressing-transgenic Arabidopsis plants, separated out the membrane fractions, subjected them to a sucrose density gradient, and detected ABCI20-GFP using an anti-GFP antibody. ABCI20-GFP was detected most strongly in fractions 3, 4, 8, and 9 (Fig. 5b). This distribution pattern was similar to that of an ER marker BiP, and different from those of the markers for the plasma membrane (AHA and PIP1), or vacuolar membrane (γ-TIP). This result indicated that ABCI20-GFP localizes to the ER membrane, consistent with the results of our microscopic analysis (Fig. 5a and Fig. S4), but different from the previously reported localization of ABCI20 based on transient over-expression in BY2 and Arabidopsis cells (Marin et al. 2006). We speculate that excess protein synthesis in the transient expression systems might have caused ectopic localization of ABCI20 to cytoplasm and nucleus, which might have masked the authentic ER-localization.
Fig. 5.
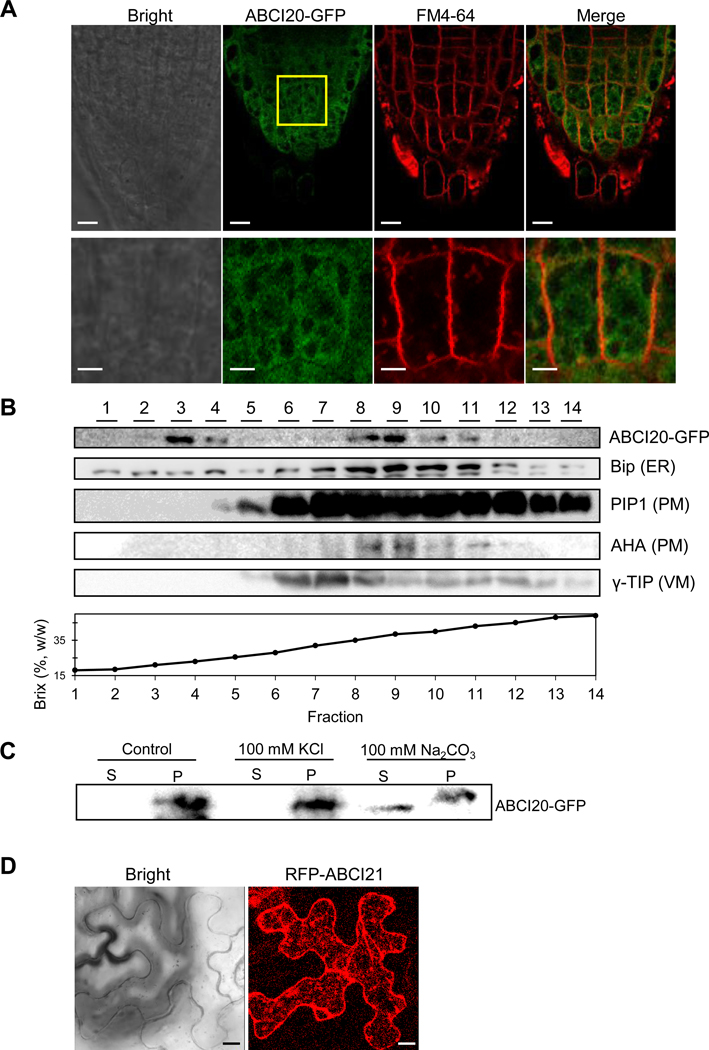
Localization of ABCI20 and ABCI21 at endoplasmic reticulum.
(a) Representative root tip images of ABCI20-GFP transgenic A. thaliana seedlings. The plasma membrane was labeled by brief staining with FM4–64. The boxed region of the root tip is magnified in the bottom panels. Scale bars = 10 μm (top panel) and 2 μm (bottom panel).
(b) Membrane fractionated by sucrose density gradient and probed with antibodies to marker proteins of different membranes.
(c) ABCI20-GFP was released to soluble fraction (S) from the membrane fractions (pellet, P) when subjected to alkaline (100 mM Na2CO3) buffers but not to high salt (100 mM KCl).
(d) Representative images of tobacco leaf epidermis cells that transiently express RFP-ABCI21. Scale bars = 10 μm.
ABCI20 does not have a transmembrane domain (TMD), according to protein topology prediction programs (TMHMM Server v. 2.0; http://www.cbs.dtu.dk/services/TMHMM-2.0/; Sonnhammer et al. 1998). To determine the manner by which ABCI20 is associated with the ER membrane, we treated total membrane fractions prepared from ABCI20-GFP expressing Arabidopsis seedlings with high concentrations of salt (KCl) and an alkalinizing chemical (Na2CO3) which are often used to dissociate peripheral proteins from the membrane. ABCI20 did not dissociate from the membrane by the high salt treatment, and only when the membrane was treated with an alkaline solution, could ABCI20 be detected in the soluble fraction (Fig. 5c). The tight association of ABCI20 with the ER membrane, despite the absence of a TMD in its own sequence, suggests that ABCI20 is a peripheral membrane protein that binds tightly with other proteins in the ER membrane.
To determine the subcellular localization of ABCI21, pro35S:RFP:ABCI21 construct was transiently expressed in the tobacco leaf epidermal cells. The fluorescence signals of RFP-ABCI21 protein was also at ER (Fig. 5d). When both ABCI20-GFP and RFP-ABCI21 were co-expressed in tobacco leaf epidermal cells, GFP and RFP signals overlapped at the ER-like network structure (Fig. S5), suggesting that these two proteins are co-localized at the ER. Taken together, these results suggest that ABCI20 and ABCI21 are localized at ER, and that ABCI20 is anchored tightly to ER-localized protein(s).
ABCI19, ABCI20, and ABCI21 are components of a large protein complex
We speculated that ABCI20 and ABCI21 interact with transmembrane proteins to be anchored at the ER membrane. To test this hypothesis, we investigated whether there is an ABCI20-containing protein complex using blue native PAGE and a home-made rabbit anti-ABCI20 antiserum that detects proteins of the expected size of ABCI20, 37 kDa (see “Materials and methods” for details and Fig. S6). Interestingly, purified anti-ABCI20 antiserum detected protein band of 300–400 kDa size, which was absent in abci20 abci21 (Fig. 6), suggesting that ABCI20 forms a large protein complex.
Fig. 6.
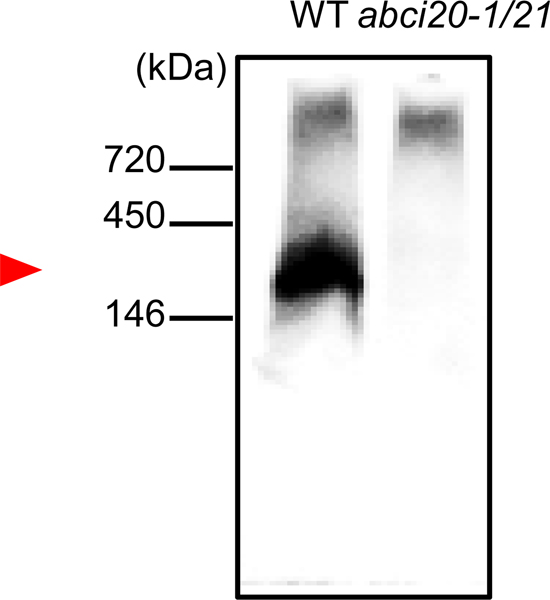
ABCI20 is a component of a large protein complex.
Whole protein extracts from the wild type and abci20–1/21 double knockout Arabidopsis seedlings were analyzed by BN-PAGE and Western blot with anti-ABC20 antibody. Anti-ABCI20 antibody detected a protein complex around 300–400 kDa (red arrowhead).
To obtain clues to which proteins might interact with ABCI20, we performed immuno-precipitation using the anti-ABCI20 antibody, subjected the pulled-down proteins to protease digestion, and identified the peptides using liquid chromatography hybrid-FT orbitrap mass spectrometer (LTQ-MS). A total of 309 peptides were read exclusively from the co-immunoprecipitation sample prepared from the wild type in this analysis (Supplemental Table 3), and among them were peptides of ABCI19 and ABCI21. The peptide sequence coverages were 75.5% for ABCI19, 47% for ABCI20, and 90.1% for ABCI21, respectively. Additionally, many interesting candidate binding partners of ABCI20 were identified, for example, all of the subunits of COPI coatomer complex, α, β, β′, γ, δ, ε and ζ (Supplemental Table 3).
ABCI20 and ABCI21 expression is induced by light in a manner dependent on HY5
ABCI19, ABCI20, and ABCI21 commonly have recognition sites of HY5 transcription factor in their promoter regions (Fig. 7a); ABCI20 contains both ACGT containing element (ACE) and G-box, the binding motifs of HY5 transcription factor (Ang et al. 1998), whereas ABCI19 and ABCI21 have only ACE (Fig. 7a). To determine whether the expression of ABCI19, ABCI20, and ABCI21 depends on HY5, the transcript levels of the three genes were examined in hy5 knockout mutant (hy5–1) and the wild types (Ler) under three different light/dark conditions. ABCI20 and ABCI21 transcript levels were higher in the wild type than in the hy5–1 mutant seedlings, and in the 30-min light exposed or light-grown seedlings than in the dark-grown ones (Fig. 7b). ABCI19 expression was very low, and its induction by light was not detectable (Fig. 7b).
Fig. 7.
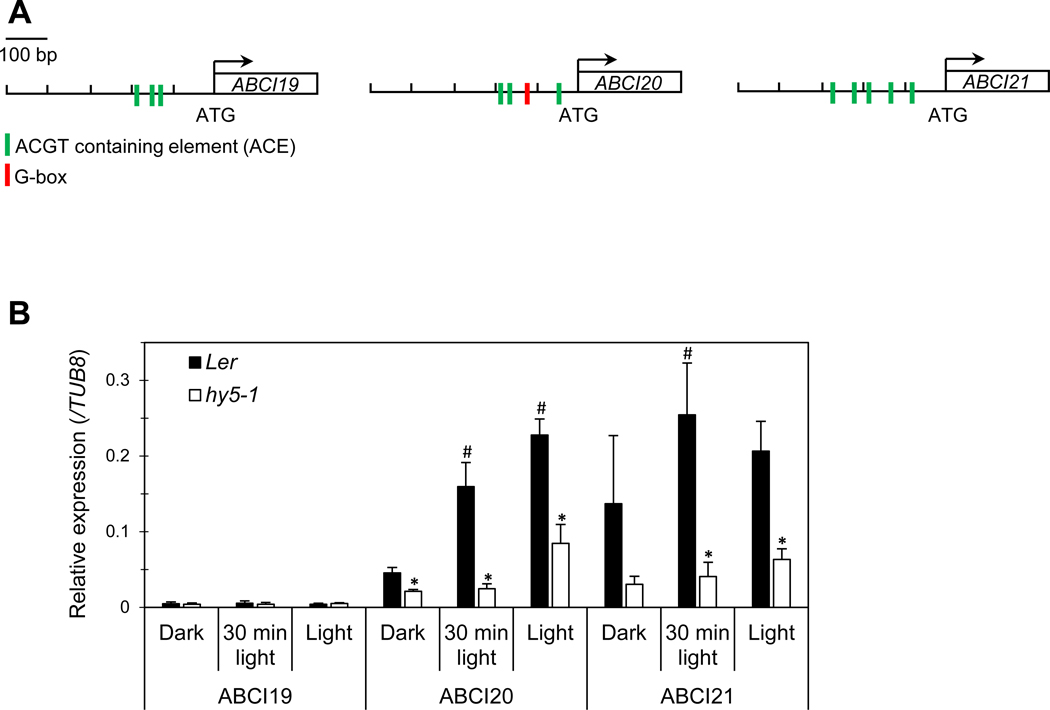
HY5-dependent induction of ABCI20 and ABCI21 upon light exposure.
(a) Promoters of ABCI19, ABCI20 and ABCI21 contain HY5-responsive elements (ACGT containing elements and/or G-box).
(b) Relative transcript levels (mean values ± SD) of ABCI19, ABCI20 and ABCI21 in hy5–1 and the wild type (Ler) under various light/dark conditions. Gene expression levels were measured by qRT-PCR and normalized by the β–Tubulin 8 expression level. Asterisks indicate significant differences between Ler and hy5–1 (p < 0.05, Student’s t test), and hashtags indicate significant differences between light-exposed samples and dark control (p < 0.05, Student’s t test; N=2, n=3).
Discussion
ABCI19/20/21 suppress the cytokinin effects on seedling growth
In this paper, we provided two lines of evidence which suggested that ABCI19/20/21 negatively regulate cytokinin response in Arabidopsis seedlings. First, knocking out of these ABCIs caused hypersensitivity of seedling growth to externally applied tZ (Figs. 3 and S3). When treated with as low as 10 nM tZ, abci19 abci20 abci21 exhibited lower shoot biomass, and shorter roots which likely resulted from reduced RAM activity, than the wild type (Figs. 3 and S3). By contrast, treatments with other hormones or hormone precursor did not induce detectable growth changes of abci19 abci20 abci21 seedlings (Fig. S1). Particularly, no phenotype of the abci19 abci20 abci21 in auxin-containing medium was remarkable since RAM activity is mainly regulated by cytokinin and auxin (Dello Ioio et al. 2007). Second, knocking out of ABCI20 and ABCI21 (abci20–1 abci21) caused the increased response of TCS:GFP in root tips (Fig. 4), indicating that type-B ARR-mediated tZ signaling was enhanced. This result was consistent with the tZ-hypersensitive inhibition of RAM activity in abci20 abci21 mutants (Fig. S3 d).
Environmental stresses often induce an increase in endogenous cytokinin level to inhibit root growth (Yang et al. 2017; Vyroubalova et al. 2009; Albrecht et al. 2017). Upon Al toxicity stress, endogenous cytokinin concentration increases, resulting in root growth inhibition, as an avoidance mechanism of Al toxicity (Yang et al. 2017). Osmotic or salinity stress induces a moderate increase of the active form of cytokinin in maize roots, and this increase lasts several days during acclimatization to stress (Vyroubalova et al. 2009). Thus, cytokinin-induced inhibition of growth is important to avoid and or acclimate to stresses. However, ABCI19, ABCI20, and ABCI21 suppressed the cytokinin-induced inhibition of growth (Fig. 3). We speculate that Arabidopsis seedlings use the three ABCI proteins to fine-tune cytokinin signaling so that it does not proceed to an extreme. Such mechanism might be necessary since environmental conditions change constantly, and young seedlings need to rapidly adapt to such changes for normal development.
BCI19, ABCI20 and ABCI21 work together at ER
In this study, we showed that ABCI20 and ABCI21 are localized at ER (Fig. 5). ABCI20-GFP protein seemed to be tightly anchored to ER, since it was detached from the membrane fraction only after treatment with a strong alkaline buffer (Fig. 5). Since ABCI20 does not contain any domain likely to span the lipid bilayer, ABCI20 most likely interacts with a membrane protein at ER. We could not identify the specific membrane protein that interacts with ABCI20, although we obtained many candidates of interacting partners (Supplemental Table 3). Interestingly, ABCI19 and ABCI21 peptides were found in the ABCI20 complex (Supplemental Table 3), suggesting that ABCI19, ABCI20 and ABCI21 interact with each other. It has been reported that many ABCI proteins are associated with and function in intracellular organelles of bacterial origins, mitochondria and plastids (Rayapuram et al. 2007; Xu et al. 2004; Roston et al. 2012; Wang et al. 2013; Fan et al. 2015) with a few exceptions (Huang et al. 2010).
Possible functions of ABCI19, ABCI20 and ABCI21
Our results indicate that ABCI19, ABCI20 and ABCI21 proteins have a function in cytokinin response under regulation by HY5. ABCI19, ABCI20 and ABCI21 have only nucleotide-binding domains (NBDs), and many ABC proteins of this kind function as a member of a multi-subunit ABC transporter complex. However, in some eukaryotic systems, these proteins are involved in other processes, such as translational elongation or transcriptional regulation (Qin et al. 1990; Liu et al. 2001). Therefore, it is necessary to consider many different possibilities for the ABCI19/20/21 function.
First, ABCI19/20/21 might function as a component of a transporter. ABCI19/20/21 may function as a subunit of a transporting complex, similarly as the ABCI13/14/15 complex (TGD). In the TGD complex, ABCI13 functions as the ATPase, ABCI14 as the permease, and ABCI15 as the substrate-binding protein, and together, they transport polar lipids from the ER to the chloroplast (Lu et al. 2007). Since abci19 abci20 abci21 exhibits cytokinin hyper-sensitive phenotype, it is plausible that ABCI19/20/21 complex reduces cytokinin amounts detected by active cytokinin receptors. ABCI19/20/21 are located in ER, and recent studies provided evidence that ER is an active place for cytokinin signaling and metabolism (Caesar et al. 2011; Lomin et al. 2015; Wulfetange et al. 2011; Niemann et al. 2018). Three cytokinin receptors, AHK2, AHK3, and AHK4, are located to the ER membrane, as well as at the plasma membrane, and endomembrane fraction showed higher cytokinin binding affinity than the plasma membrane (Wulfetange et al. 2011). In addition, ER is the place for cytokinin metabolism (Niemann et al. 2018). Thus, ER might be the site of active cytokinin binding to receptors and/or degradation of cytokinin to reduce cytokinin response in the cells. Thus both importers and exporters of cytokinin are expected at ER membrane. Together with their membrane partner(s), ABCI19/20/21 might function as a cytokinin transporter, reducing cytokinin binding to the active receptors of cytokinin.
Second, ABCI19/20/21 might regulate the localization of proteins important for cytokinin signal transduction. This idea is based on our co-immunoprecipitation experiment where we found all seven subunits of coatomers of COPI complex potentially associated with ABCI20 (Supplemental Table 3). Since all subunits are found in the assay, it is difficult to disregard them as false positives. Thus we speculate that Arabidopsis cells regulate their sensitivity to cytokinin by regulating the localization of receptors, or enzymes that metabolize cytokinin. Since ABCI19/20/21 have ATPase domains, they might function as ATPases, providing the energy necessary to complete the retrograde vesicle trafficking mediated by COPI.
ABCI19/20/21 function in downstream of HY5
ABCI20 and ABCI21 expression was largely dependent on HY5 (Fig. 7), a central regulator of photomorphogenesis. The promoter regions of ABCI19/20/21 have HY5 binding motifs, and transcripts levels of ABCI20 and ABCI21 were significantly reduced in hy5 mutant plants (Fig. 7), consistent with the previous finding of ABCI20 and ABCI21 as candidates of direct targets of HY5 (Lee et al. 2007). Our results revealed that the HY5-driven expression of ABCI20 and ABCI21 negatively regulates the effects of exogenous cytokinin, suggesting a crosstalk between HY5-mediated and cytokinin-mediated signaling processes. Such crosstalk might be necessary to optimize the growth of seedlings, which have to establish themselves as photoautotropic organisms under the pressure of many changes in external and internal conditions. Although not much is known on such crosstalk, cytokinin was reported to reduce the nuclear accumulation of CONSTITUTIVE PHOTO-MORPHOGENIC1 (COP1), stabilizing HY5 protein (Vandenbussche et al. 2007). Thus we speculate that ABCI20 and ABCI21, and possibly ABCI19, too, are involved in the crosstalk between HY5 and cytokinin, which regulates seedling growth and development. The mechanism of the crosstalk remains to be determined in future.
Supplementary Material
Key message.
Non-intrinsic ABC proteins ABCI20 and ABCI21 are induced to express by light under HY5 regulation, localized at ER, and ameliorate cytokinin-driven growth inhibition in young seedlings of Arabidopsis thaliana.
Acknowledgments
This research was supported by Basic Science Research Program NRF-2018R1A2A1A05018173 (to Y.L.) through the National Research Foundation of Korea funded by the Ministry of Science and ICT (Information and Communication Technology), Dr. Hwang’s grant number (to J.-U.H.,), a Grant-in-Aid for Scientific Research (A) (26252011) from Japan (to M.M.), and Professor Zhao’s grant number.
Footnotes
Compliance with ethical standards
Conflict of interest: The authors declare no conflict of interest.
References
- Albrecht T, Argueso CT (2017) Should I fight or should I grow now? The role of cytokinins in plant growth and immunity and in the growth-defence trade-off. Ann Bot 119:725–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali T, Bednarska J, Vassilopoulos S, Tran M, Diakonov IA, Ziyadeh-Isleem A, Guicheney P, Gorelik J, Korchev YE, Reilly MM, Bitoun M, Shevchuk A (2019) Correlative SICM-FCM reveals changes in morphology and kinetics of endocytic pits induced by disease-associated mutations in dynamin. Faseb j 33:8504–8518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang LH, Chattopadhyay S, Wei N, Oyama T, Okada K, Batschauer A, Deng XW (1998) Molecular interaction between COP1 and HY5 defines a regulatory switch for light control of Arabidopsis development. Mol Cell 1:213–222 [DOI] [PubMed] [Google Scholar]
- Caesar K, Thamm AM, Witthoft J, Elgass K, Huppenberger P, Grefen C, Harter K (2011) Evidence for the localization of the Arabidopsis cytokinin receptors AHK3 and AHK4 in the endoplasmic reticulum. J Exp Bot 62:5571–5580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CS, Li YH, Chen LT, Chen WC, Hsieh WP, Shin J, Wu SH (2008) LZF1, a HY5-regulated transcriptional factor, functions in Arabidopsis de-etiolation. Plant J 54:205–219 [DOI] [PubMed] [Google Scholar]
- Chattopadhyay S, Ang LH, Puente P, Deng XW, Wei N (1998) Arabidopsis bZIP protein HY5 directly interacts with light-responsive promoters in mediating light control of gene expression. Plant Cell 10:673–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Zhang J, Neff MM, Hong SW, Zhang H, Deng XW, Xiong L (2008) Integration of light and abscisic acid signaling during seed germination and early seedling development. Proc Natl Acad Sci U S A 105:4495–4500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MD, Grossniklaus U (2003) A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiology 133:462–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dello Ioio R, Linhares FS, Scacchi E, Casamitjana-Martinez E, Heidstra R, Costantino P, Sabatini S (2007) Cytokinins determine Arabidopsis root-meristem size by controlling cell differentiation. Curr Biol 17:678–682 [DOI] [PubMed] [Google Scholar]
- Do THT, Martinoia E, Lee Y (2018) Functions of ABC transporters in plant growth and development. Curr Opin Plant Biol 41:32–38 [DOI] [PubMed] [Google Scholar]
- Fan J, Zhai Z, Yan C, Xu C (2015) Arabidopsis TRIGALACTOSYLDIACYLGLYCEROL5 interacts with TGD1, TGD2, and TGD4 to facilitate lipid transfer from the endoplasmic reticulum to plastids. Plant Cell 27:2941–2955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favory JJ, Stec A, Gruber H, Rizzini L, Oravecz A, Funk M, Ulm R (2009) Interaction of COP1 and UVR8 regulates UV-B-induced photomorphogenesis and stress acclimation in Arabidopsis. Embo J 28:591–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangappa SN, Botto JF (2016) The multifaceted roles of HY5 in plant growth and development. Mol Plant 9:1353–1365 [DOI] [PubMed] [Google Scholar]
- Gao X, Chen J, Dai X, Zhang D, Zhao Y (2016) An effective strategy for reliably isolating heritable and Cas9-free Arabidopsis mutants generated by CRISPR/Cas9-mediated genome editing. Plant Physiol 171:1794–1800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Zhao Y (2014) Self-processing of ribozyme-flanked RNAs into guide RNAs in vitro and in vivo for CRISPR-mediated genome editing. J Integr Plant Biol 56:343–349 [DOI] [PubMed] [Google Scholar]
- Hosoda K, Imamura A, Katoh E, Hatta T, Tachiki M, Yamada H, Yamazaki T (2002) Molecular structure of the GARP family of plant Myb-related DNA binding motifs of the Arabidopsis response regulators. Plant Cell 14:2015–2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CF, Yamaji N, Ma JF (2010) Knockout of a bacterial-type ATP-binding cassette transporter gene, AtSTAR1, results in increased aluminum sensitivity in Arabidopsis. Plant Physiol 153:1669–1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JU, Yim S, Do THT, Kang J, Lee Y (2018) Arabidopsis thaliana Raf22 protein kinase maintains growth capacity during postgerminative growth arrest under stress. Plant Cell Environ 41:1565–1578 [DOI] [PubMed] [Google Scholar]
- Imamura A, Kiba T, Tajima Y, Yamashino T, Mizuno T (2003) In vivo and in vitro characterization of the ARR11 response regulator implicated in the His-to-Asp phosphorelay signal transduction in Arabidopsis thaliana. Plant Cell Physiol 44:122–131 [DOI] [PubMed] [Google Scholar]
- Kang J, Park J, Choi H, Burla B, Kretzschmar T, Lee Y, Martinoia E (2011) Plant ABC transporters. Arabidopsis Book 9:e0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YY, Choi H, Segami S, Cho HT, Martinoia E, Maeshima M, Lee Y (2009) AtHMA1 contributes to the detoxification of excess Zn(II) in Arabidopsis. Plant J 58:737–753 [DOI] [PubMed] [Google Scholar]
- Kobae Y, Sekino T, Yoshioka H, Nakagawa T, Martinoia E, Maeshima M (2006) Loss of AtPDR8, a plasma membrane ABC transporter of Arabidopsis thaliana, causes hypersensitive cell death upon pathogen infection. Plant Cell Physiol 47:309–318 [DOI] [PubMed] [Google Scholar]
- Koornneef M, Rolff E, Spruit C (1980) Genetic control of light-inhibited hypocotyl elongation in Arabidopsis thaliana (L.) Heynh. Zeitschrift für Pflanzenphysiologie 100:147–160 [Google Scholar]
- Kudo T, Makita N, Kojima M, Tokunaga H, Sakakibara H (2012) Cytokinin activity of cis-zeatin and phenotypic alterations induced by overexpression of putative cis-Zeatin-O-glucosyltransferase in rice. Plant Physiol 160:319–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen PB, Geisler MJ, Jones CA, Williams KM, Cancel JD (2005) ALS3 encodes a phloem-localized ABC transporter-like protein that is required for aluminum tolerance in Arabidopsis. Plant J 41:353–363 [DOI] [PubMed] [Google Scholar]
- Lee J, He K, Stolc V, Lee H, Figueroa P, Gao Y, Deng XW (2007) Analysis of transcription factor HY5 genomic binding sites revealed its hierarchical role in light regulation of development. Plant Cell 19:731–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, Lee K, Lee J, Noh EW, Lee Y (2005) AtPDR12 contributes to lead resistance in Arabidopsis. Plant Physiol 138:827–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HY, Chiang YC, Pan J, Chen J, Salvadore C, Audino DC, Denis CL (2001) Characterization of CAF4 and CAF16 reveals a functional connection between the CCR4-NOT complex and a subset of SRB proteins of the RNA polymerase II holoenzyme. J Biol Chem 276:7541–7548 [DOI] [PubMed] [Google Scholar]
- Lomin SN, Krivosheev DM, Steklov MY, Arkhipov DV, Osolodkin DI, Schmulling T, Romanov GA (2015) Plant membrane assays with cytokinin receptors underpin the unique role of free cytokinin bases as biologically active ligands. J Exp Bot 66:1851–1863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Xu C, Awai K, Jones AD, Benning C (2007) A small ATPase protein of Arabidopsis, TGD3, involved in chloroplast lipid import. J Biol Chem 282:35945–35953 [DOI] [PubMed] [Google Scholar]
- Marin E, Divol F, Bechtold N, Vavasseur A, Nussaume L, Forestier C (2006) Molecular characterization of three Arabidopsis soluble ABC proteins which expression is induced by sugars. Plant Science 171:84–90 [Google Scholar]
- Mitchell AL, Attwood TK, Babbitt PC, Blum M, Bork P, Bridge A, Finn RD (2019) InterPro in 2019: improving coverage, classification and access to protein sequence annotations. Nucleic Acids Res 47:D351–d360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller B, Sheen J (2008) Cytokinin and auxin interaction in root stem-cell specification during early embryogenesis. Nature 453:1094–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemann MCE, Weber H, Hluska T, Leonte G, Anderson SM, Novak O, Werner T (2018) The cytokinin oxidase/dehydrogenase CKX1 is a membrane-bound protein requiring homooligomerization in the endoplasmic reticulum for its cellular activity. Plant Physiol 176:2024–2039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura K, Goh T, Toyokura K, Kasahara H, Takebayashi Y, Mimura T, Fukaki H (2013) GNOM/FEWER ROOTS is required for the establishment of an auxin response maximum for Arabidopsis lateral root initiation. Plant Cell Physiol 54:406–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin SL, Xie AG, Bonato MC, McLaughlin CS (1990) Sequence analysis of the translational elongation factor 3 from Saccharomyces cerevisiae. J Biol Chem 265:1903–1912 [PubMed] [Google Scholar]
- Rasmussen A, Mason MG, De Cuyper C, Brewer PB, Herold S, Agusti J, Beveridge CA (2012) Strigolactones suppress adventitious rooting in Arabidopsis and pea. Plant Physiol 158:1976–1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayapuram N, Hagenmuller J, Grienenberger JM, Giege P, Bonnard G (2007) AtCCMA interacts with AtCcmB to form a novel mitochondrial ABC transporter involved in cytochrome c maturation in Arabidopsis. J Biol Chem 282:21015–21023 [DOI] [PubMed] [Google Scholar]
- Roston RL, Gao J, Murcha MW, Whelan J, Benning C (2012) TGD1, −2, and −3 proteins involved in lipid trafficking form ATP-binding cassette (ABC) transporter with multiple substrate-binding proteins. J Biol Chem 287:21406–21415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai H, Aoyama T, Oka A (2000) Arabidopsis ARR1 and ARR2 response regulators operate as transcriptional activators. Plant J 24:703–711 [DOI] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikholeslam SN, Weeks DP (1987) Acetosyringone promotes high efficiency transformation of Arabidopsis thaliana explants by Agrobacterium tumefaciens. Plant Mol Biol 8:291–298 [DOI] [PubMed] [Google Scholar]
- Sonnhammer EL, von Heijne G, Krogh A (1998) A hidden Markov model for predicting transmembrane helices in protein sequences. Proc Int Conf Intell Syst Mol Biol 6:175–182 [PubMed] [Google Scholar]
- Sun J, Ma Q, Mao T (2015) Ethylene regulates the Arabidopsis microtubule-associated protein WAVE-DAMPENED2-LIKE5 in etiolated hypocotyl elongation. Plant Physiol 169:325–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Nakamura Y, Asami T, Yoshida S, Matsuo T, Okamoto S (2003) Physiological roles of brassinosteroids in early growth of Arabidopsis: brassinosteroids have a synergistic relationship with gibberellin as well as auxin in light-grown hypocotyl elongation. Journal of Plant Growth Regulation 22:259–271 [Google Scholar]
- Vandenbussche F, Habricot Y, Condiff AS, Maldiney R, Van der Straeten D, Ahmad M (2007) HY5 is a point of convergence between cryptochrome and cytokinin signalling pathways in Arabidopsis thaliana. Plant J 49:428–441 [DOI] [PubMed] [Google Scholar]
- Verrier PJ, Bird D, Burla B, Dassa E, Forestier C, Geisler M, Theodoulou FL (2008) Plant ABC proteins--a unified nomenclature and updated inventory. Trends Plant Sci 13:151–159 [DOI] [PubMed] [Google Scholar]
- Vyroubalova S Vaclavikova K, Tureckova V, Novak O, Smehilova M, Hluska T, Ohnoutkova L, Frebort I, Galuszka P (2009) Characterization of new maize genes putatively involved in cytokinin metabolism and their expression during osmotic stress in relation to cytokinin levels. Plant Physiol 151:433–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Anderson NS, Benning C (2013) The phosphatidic acid binding site of the Arabidopsis trigalactosyldiacylglycerol 4 (TGD4) protein required for lipid import into chloroplasts. J Biol Chem 288:4763–4771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulfetange K, Lomin SN, Romanov GA, Stolz A, Heyl A, Schmulling T (2011) The cytokinin receptors of Arabidopsis are located mainly to the endoplasmic reticulum. Plant Physiol 156:1808–1818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Li J, Gangappa SN, Hettiarachchi C, Lin F, Andersson MX, Holm M (2014) Convergence of light and ABA signaling on the ABI5 promoter. PLoS Genet 10:e1004197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XM, Moller SG (2004) AtNAP7 is a plastidic SufC-like ATP-binding cassette/ATPase essential for Arabidopsis embryogenesis. Proc Natl Acad Sci U S A 101:9143–9148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZB, Liu G, Liu J, Zhang B, Meng W, Muller B, Ding Z (2017) Synergistic action of auxin and cytokinin mediates aluminum-induced root growth inhibition in Arabidopsis. EMBO Rep 18:1213–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo YJ, Lee HK, Han W, Kim DH, Lee MH, Jeon J, Hwang I (2016) Interactions between transmembrane helices within monomers of the aquaporin AtPIP2;1 play a crucial role in tetramer formation. Mol Plant 9:1004–1017 [DOI] [PubMed] [Google Scholar]
- Zhong C, Xu H, Ye S, Wang S, Li L, Zhang S, Wang X (2015) Gibberellic Acid-Stimulated Arabidopsis6 serves as an integrator of gibberellin, abscisic acid, and glucose signaling during seed germination in Arabidopsis. Plant Physiol 169:2288–2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong C, Xu H, Ye S, Zhang S, Wang X (2016) Arabidopsis seed germination assay with gibberellic acid. Bio-Protocol 6:e2005 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


