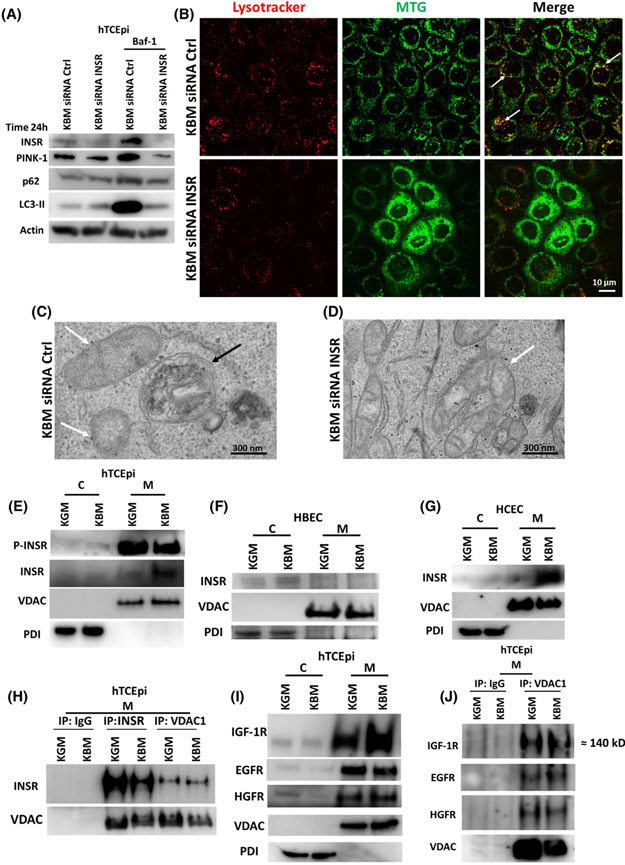
FIGURE 9.
INSR regulates mitophagy and accumulates in mitochondria in hTCEpi cells. hTCEpi cells were treated with siRNA oligonucleotides targeting INSR in KBM for 24 hours with or without 10 nM of bafilomycin (Baf-1). A, Immunoblotting for PINK-1, p62 and LC3 was used to show autophagic flux. Actin was used as loading control and blotting for INSR was used to confirm knockdown. A siRNA non-targeting oligonucleotide was used as transfection control. INSR knockdown increased LC3-II and p62 but decreased PINK-1. Baf-1 blocked autophagic flux and showed accumulation of LC3-II, p62 and PINK-1. Accumulation was completely blocked by INSR knockdown. n = 3. B, MTG (green) was used to stain mitochondria and LysoTracker (red) to stain lysosomes. In KBM, there was co-localization of MTG and LysoTracker (white arrows). INSR knockdown blocked co-localization between MTG and LysoTracker, decreased the number of lysosomes, and induced mitochondrial fragmentation. Scale bar: 10 μm. n = 3. C,D, TEM was used to show the absence of autophagolysosomes containing mitochondria in hTCEpi cells after INSR knockdown in KBM. C, Autophagolysosomes were visible in the siRNA non-targeting control (white arrows: mitochondria; black arrows: autophagolysosome). D, Autophagosomes and autophagolysosomes were absent following siRNA for INSR. Scale bar: 300 nm. hTCEpi cells, HCECs, and HBECs were cultured in growth (KGM) and basal media (KBM) for 48 hours and separated into cytosolic (C) and mitochondrial fractions (M). E, Immunoblotting for INSR in hTCEpi cells showed accumulation of the receptor in mitochondria in KBM. P-INSR immunoblotting also showed a decrease in phosphorylation of INSR in the mitochondrial fraction. E, The presence of INSR in the mitochondrial fraction was confirmed in HCECs. F, In HBECs, INSR was undetectable in the mitochondria. VDAC was used as a loading control for the mitochondrial fraction and PDI for the cytosolic fraction. G, INSR was also found in the mitochondrial fraction in HCECs. H, hTCEpi cells were cultured in growth (KGM) and basal media (KBM) for 48 hours and subject to mitochondrial fractionation (M). Samples were immunoprecipitated with an antibody specific for the homo-tetrameric INSR, VDAC1, and an IgG control. Subsequent immunoblotting for INSR and pan-VDAC confirmed pull down and indicated the presence of an interaction between INSR and VDAC. I, Immunoblotting for IGF-1R, EGFR, and HGFR confirmed the presence of these receptors in the mitochondria. J, Mitochondrial fractions isolated from hTCEpi cells were immunoprecipitated using antibodies for IgG and VDAC1. Immunoblotting confirmed mitochondrial localization and interactions with IGF-1R, EGFR, and HGFR. n = 3