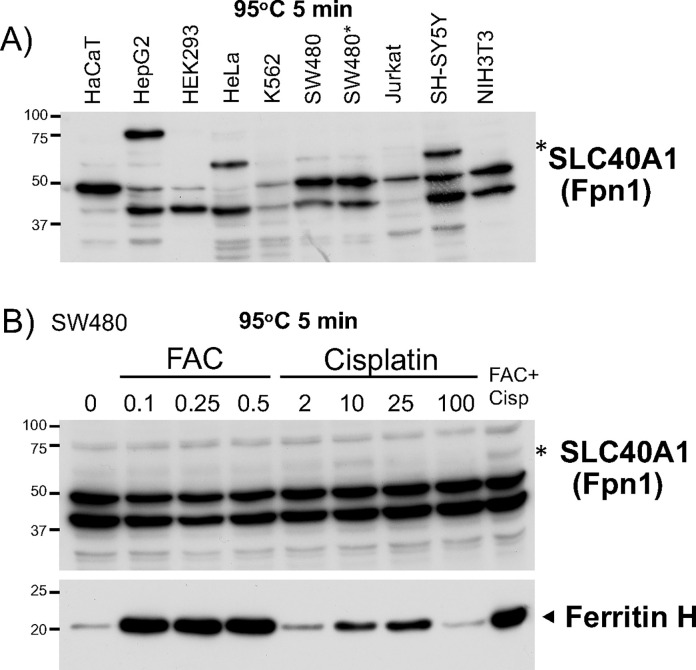
Fig 1. Heated samples for Fpn1 (SLC40A1) western blotting.
A) 25ug of whole cell lysates (WCLs) prepared from indicated cells (*two different preparations of SW480 WCLs) were subjected to Fpn1 western blotting as detailed in the Materials and Methods. This experiment used sample loading buffer containing 5% β-mercaptoethanol, heated at 95°C for 5 min, anti-Fpn1 antibody (Novus, rabbit polyclonal, 1,000-fold diluted with 1% BSA in T/TBS) at 4°C overnight followed by anti-rabbit IgG-HRP conjugated (8,000-fold diluted with 1% BSA in T/TBS) at room temperature for 1.5hr. Unless otherwise noted, the rest of western blots in this study were performed in the same procedures with or without sample heating prior to gel loading. B) SW480 cells were treated with 0.1–0.5mM FAC (ferric ammonium citrate), 2-100ug/ml cisplatin, or FAC 0.25mM plus cisplatin 10ug/ml for 18hr. 20ug of WCLs were subjected to Western blotting with anti-Fpn1 antibody (top). The membrane was soaked in the stripping solution at room temperature for 1hr, washed with T/TBS, and incubated with anti-ferritin H mouse monoclonal antibody (sc-135667, Santa Cruz Biotechnology). In A) and B), the asterisks indicate the predicted position (63 kDa) of the endogenous Fpn1. The experiments were repeated three times and the representative western blots are shown.