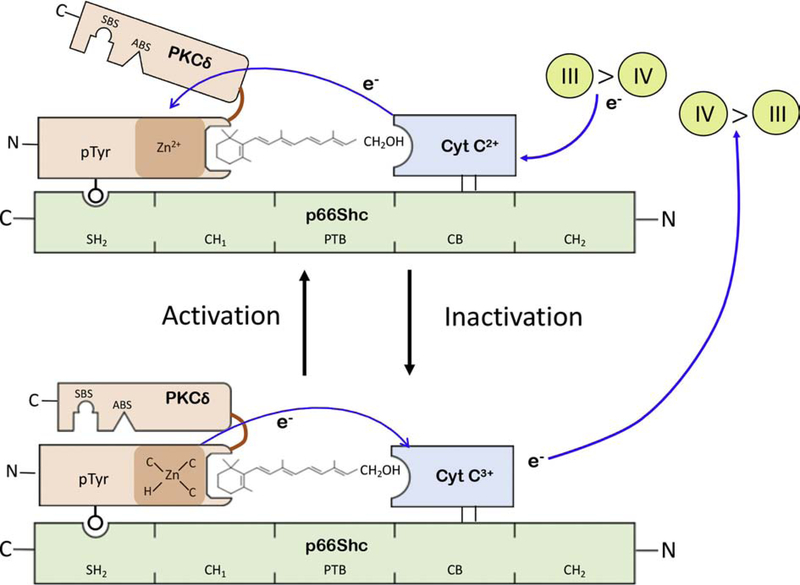
Figure 1: Model of the PKCδ/retinol signalosome in mitochondria.
The PKCδ signalosome is shown as an assemblage of two proteins, PKCδ (in brown) and cytochrome c (in blue), on the p66Shc platform (in green). The phosphotyrosine residue 332 of PKCδ binds the SH2 domain of p66Shc, and cytochrome c (cyt c) connects to the p66Shc CB (cytochrome c binding domain) by hydrophobic interaction. Retinol occupies dedicated binding pockets on PKCδ which coincides with the two cysteine-rich activation domains (CRD) of this kinase, only one them being shown for clarity. The CRD contains two Zn2+ ions, configured into a rigid zinc-finger (also known as RING Finger) by chelation of each Zn cation with 3 cysteinyl-sulfhydryl anions (C), and one histidinyl anion (H). In mitochondria PKCδ is activated by oxidation of RING-finger cysteines, presumably generating one or two cystine residues, depending on whether one or both zinc-coordination centers are oxidized. Oxidation entails the transfer of at least one pair of electrons (e-) from cysteines to cyt c3+. Electron transfer is catalyzed by vitamin A (retinol) which bridges the gap between the PKCδ CRD and cyt c. In consequence to RING-finger oxidation the CRD undergoes a local structural change resulting, when amplified by allosteric means, in the retraction of the regulatory domain from the catalytic domain and providing access to substrate binding (SBS) and ATP binding sites (ABS).
The unfolding process from globular, inactive PKCδ to the active enzyme is postulated to be reversible. This step presumably entails the reduction of cystines (generated during the activation step) by vitamin A-catalyzed transfer of electrons from cyt c2+ that accumulates in the IMS during periods of high respiratory activity. It is presently unknown whether the restoration of the RING-finger, along with the large-scale restoration of the globular protein, occurs spontaneously or, as seems likely, involves auxiliary chaperones. It is noteworthy that the silencing of the bacterial HSP33 protein refoldase is assisted by the DNAK/DNAJ/GrpE auxiliary chaperone machinery. Refolding PKCδ might require orthologous auxiliary chaperones.