Fig. 5.
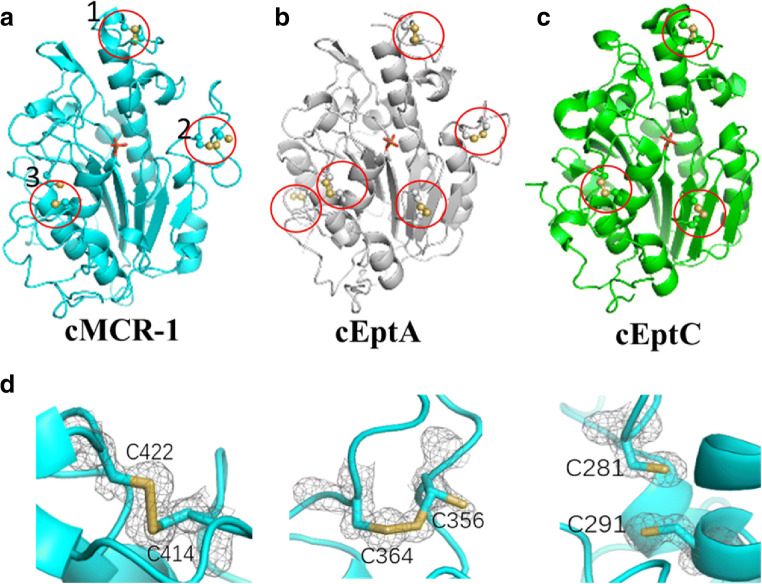
Multiple pairs of cysteine residues are present in cMCR-1, cEptA, and cEptC. a–c Multiple pairs of cysteine residues are common features in phosphoethanolamine transferase family proteins, including cMCR-1, cEptA, and cEptC, which have 3, 5, and 3 pairs, respectively. Disulfides (yellow) are highlighted by red circles. d In cMCR-1, the 3 pairs of cysteine residues exhibit 3 different oxidation states, where all, some, or none are oxidized. Cys281 and Cys291 are not observed in their oxidized form. Cys356 and Cys364 are observed in partially oxidized forms, while Cys414 and Cys422 are observed in their fully oxidized form. Different states of disulfide bond formation are also observed in cEptA